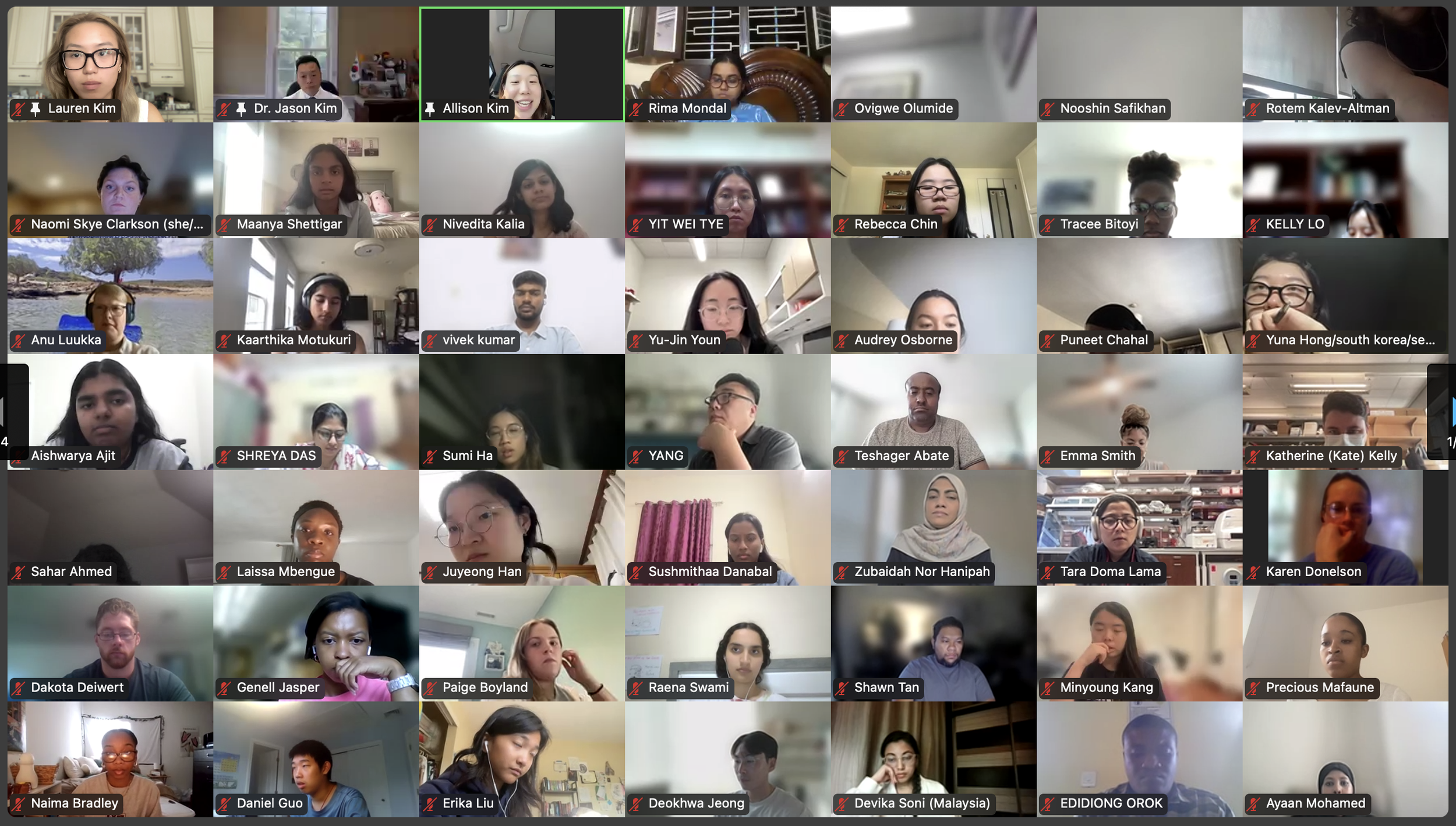
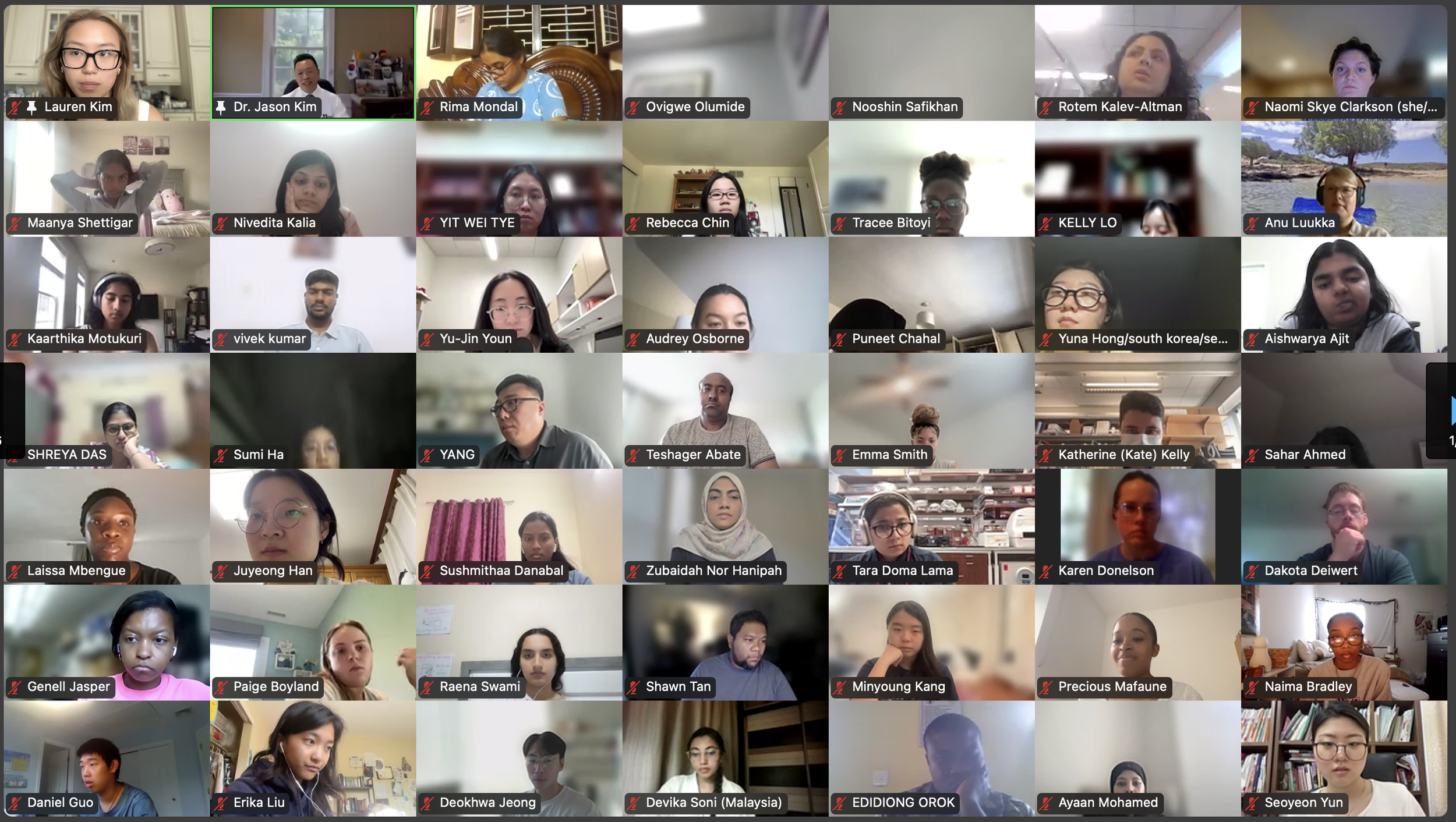
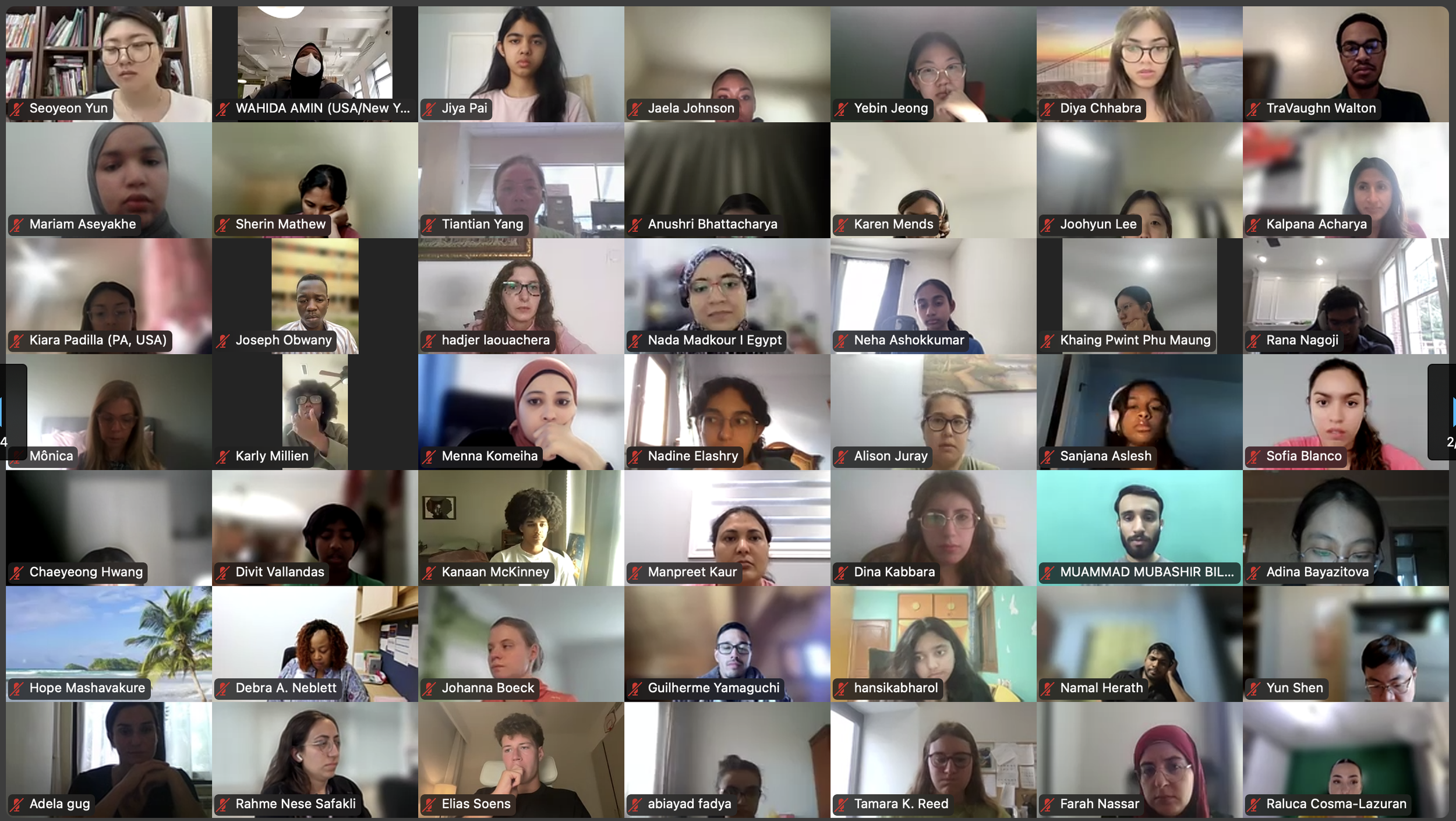
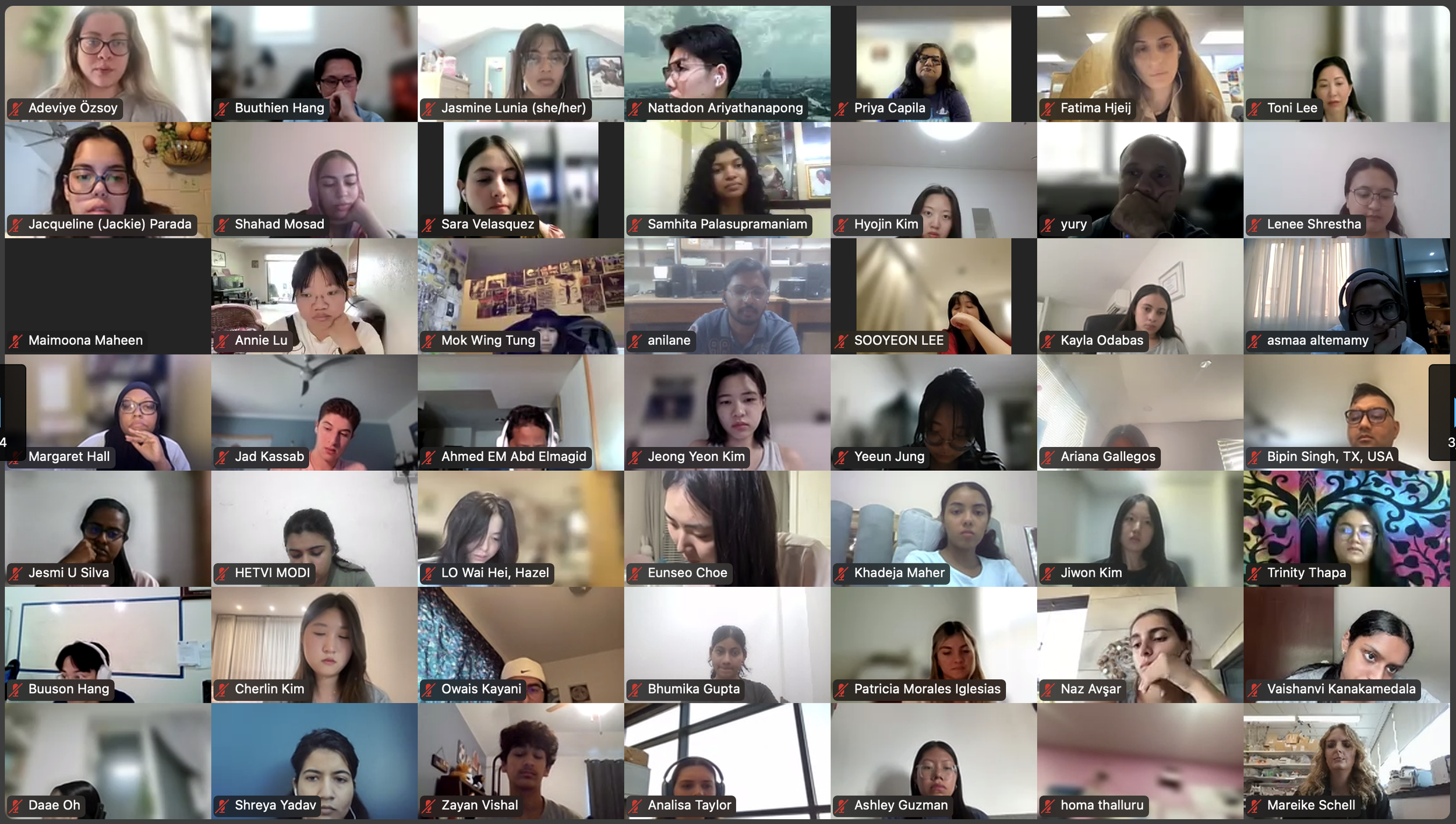
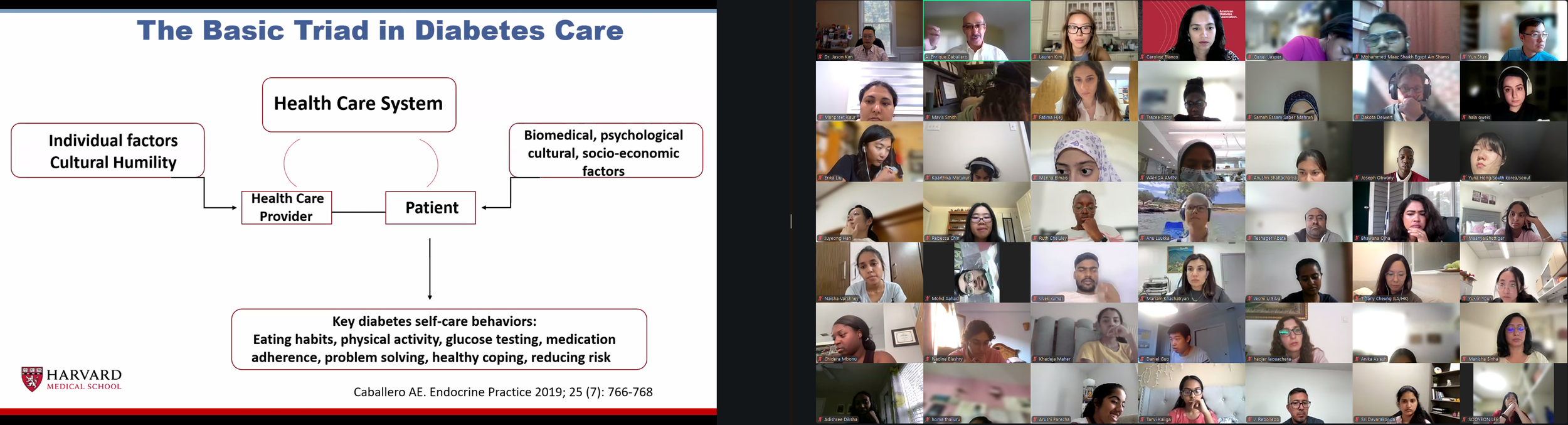
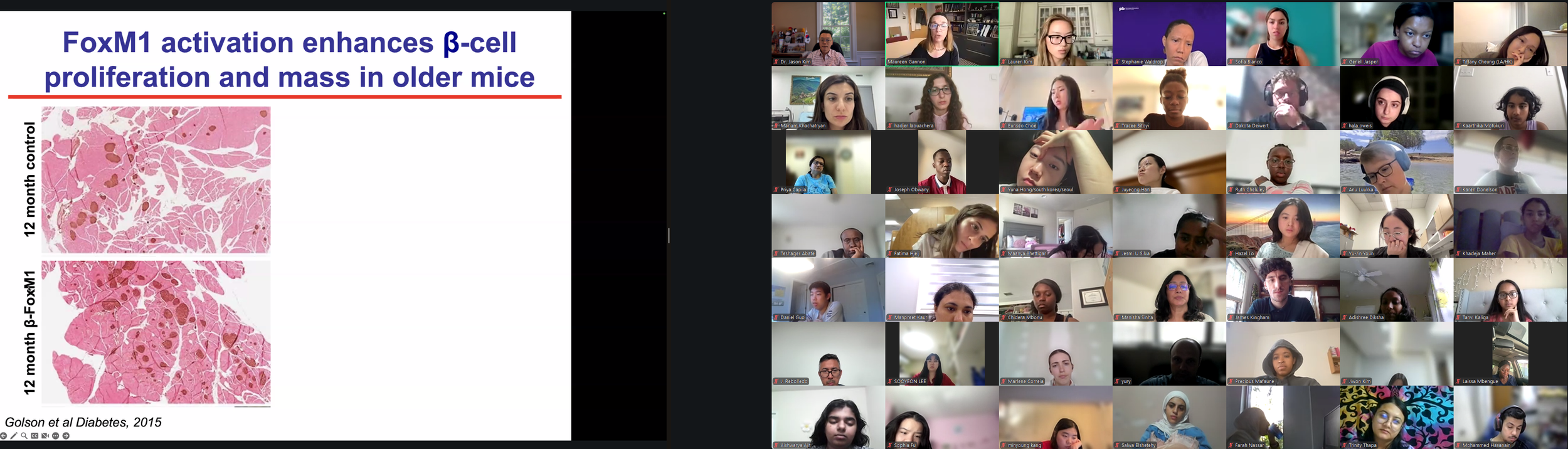
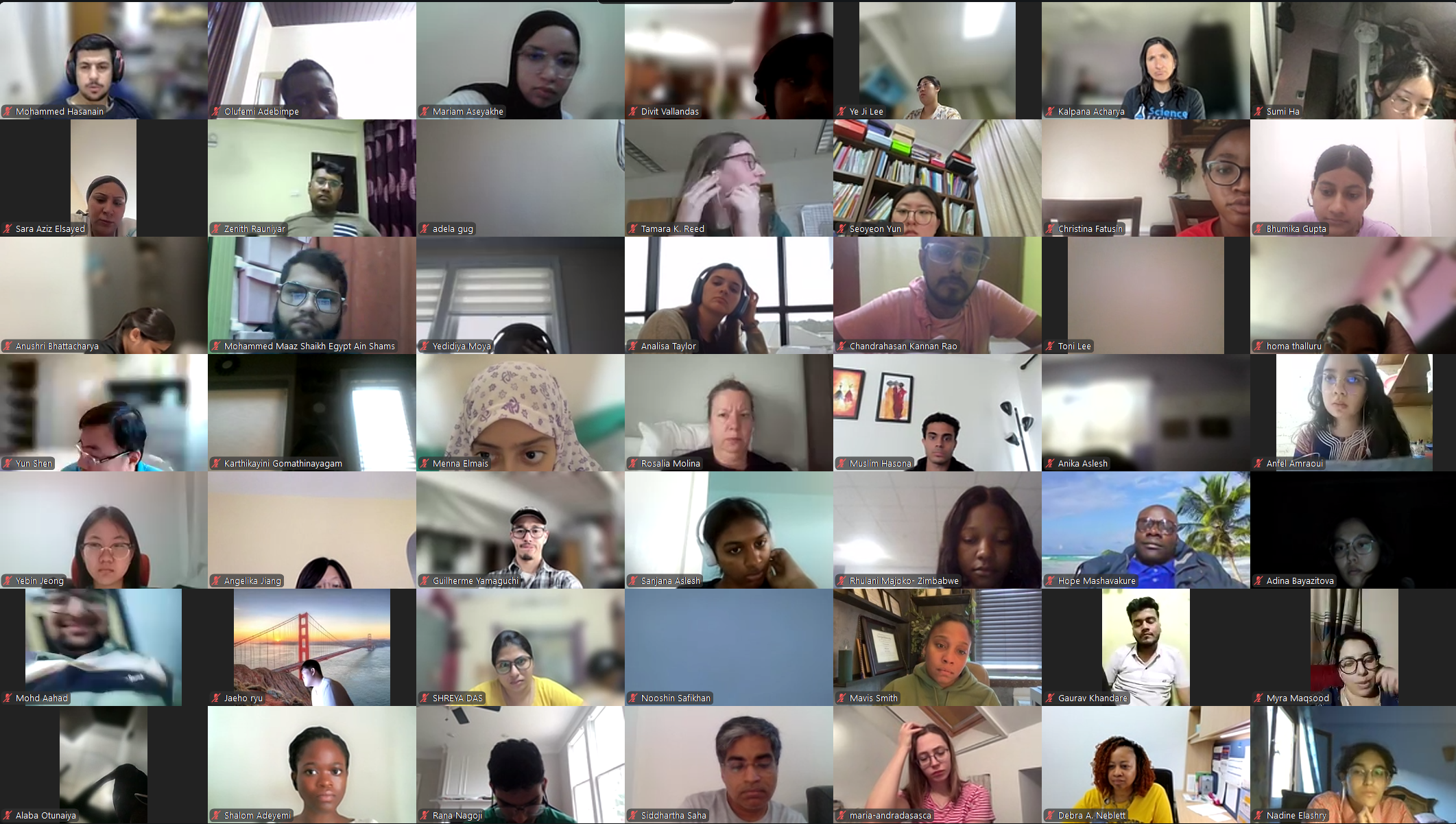
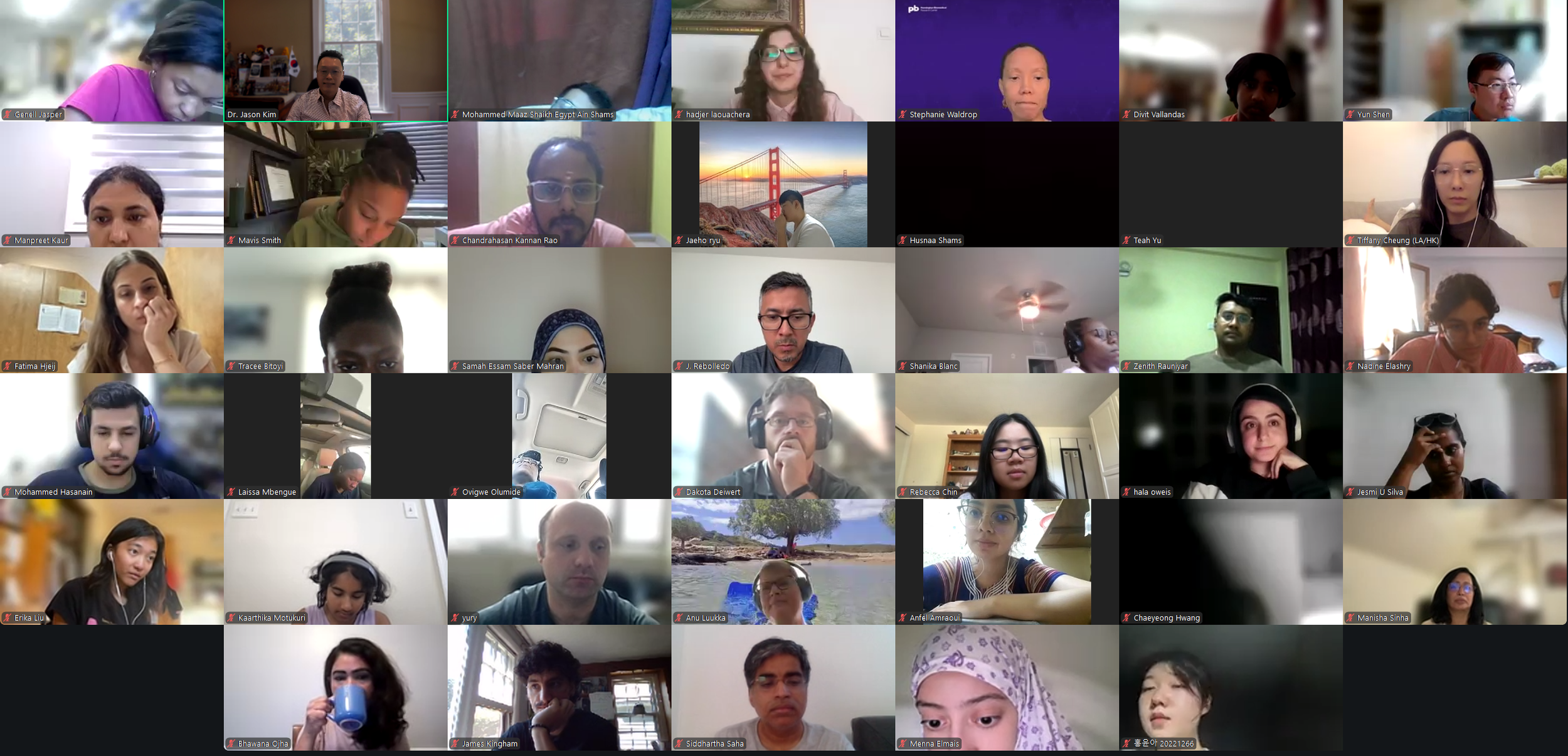
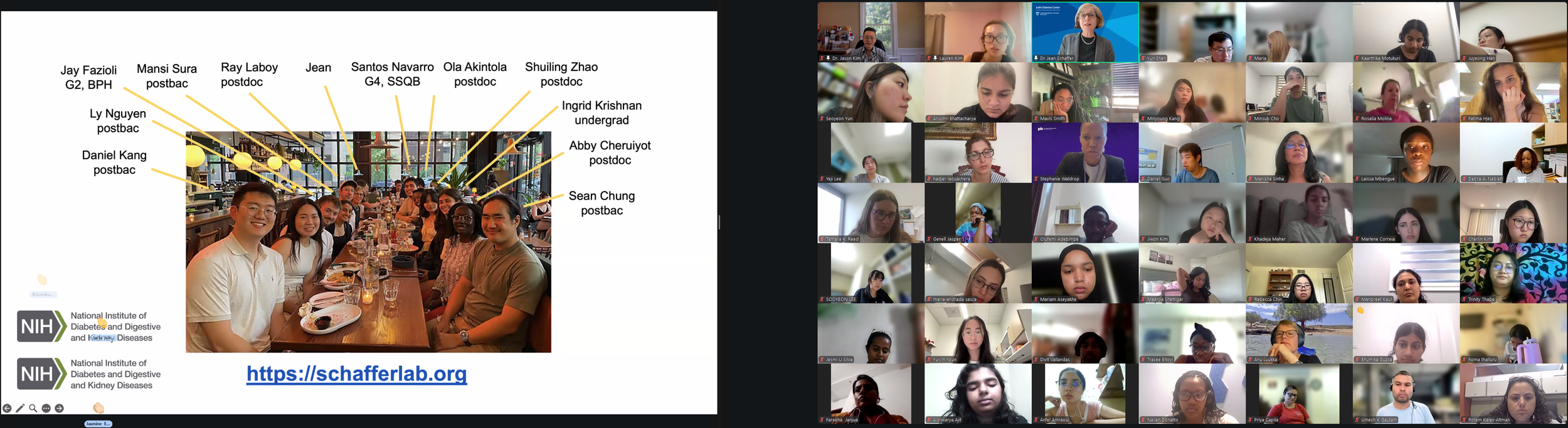
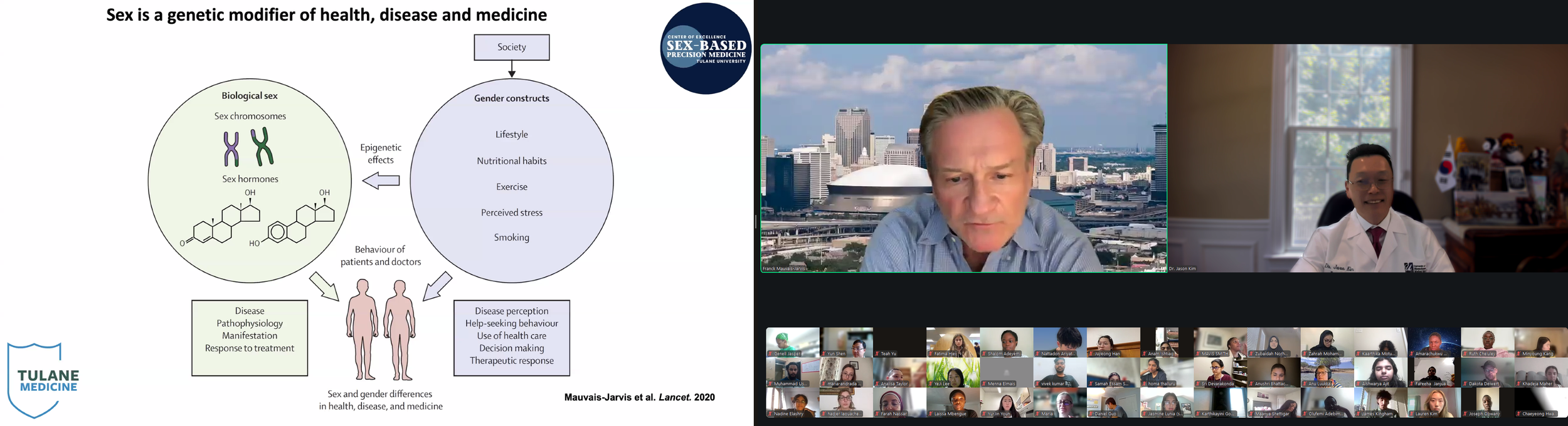
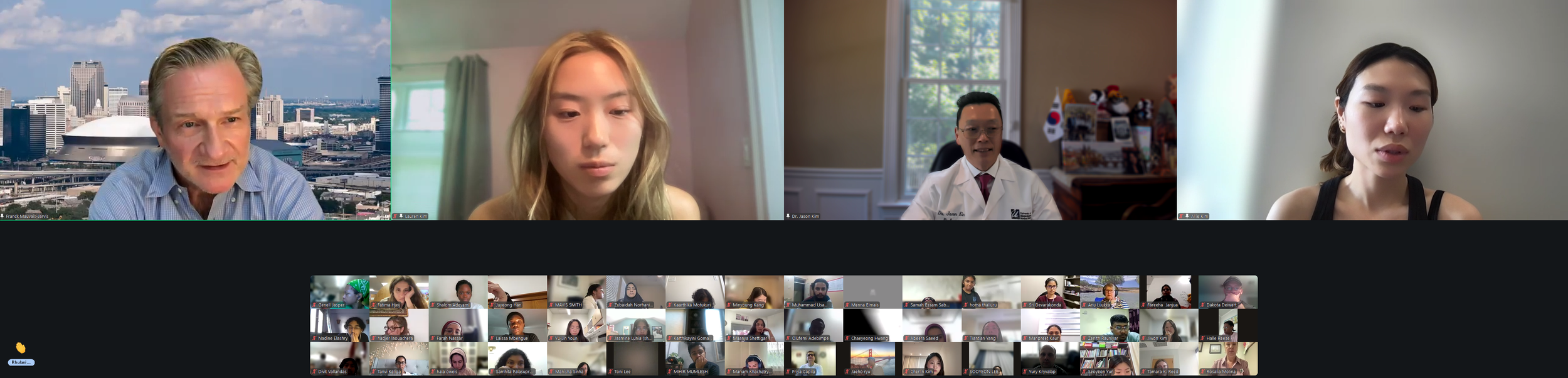
The Diabetes Virtual Summer Camp 2025 (DVSC25) has 984 registered interns, representing over 500 institutions from 70 countries.
Our DVSC25 interns come from Algeria, Armenia, Australia, Bangladesh, Belarus, Belgium, Brazil, Cameroon, Canada, Chile, China, Colombia, Cyprus, Ecuador, Egypt, England, Ethiopia, Finland, France, Georgia, Germany, Ghana, Greece, Hong Kong, Hungary, India, Indonesia, Iraq, Ireland, Italy, Jordan, Kazakhstan, Kenya, Kuwait, Lebanon, Malawi, Malaysia, Mexico, Morocco, Myanmar, Nepal, Netherlands, Nigeria, Pakistan, Palestinian Territories, Philippines, Portugal, Qatar, Romania, Russia, Saudi Arabia, Scotland, Serbia, Singapore, South Korea, Spain, Sri Lanka, Sudan, Sweden, Syria, Tanzania, Thailand, Turkey, Uganda, Ukraine, United Arab Emirates, Vietnam, Zambia, Zimbabwe, and the United States of America.
The 351 interns from the United States represent 38 states: Alaska (2), Alabama (6), Arkansas (2), Arizona (6), California (53), Colorado (1), Connecticut (4), District of Columbia (1), Florida (5), Georgia (7), Illinois (19), Indiana (9), Kansas (2), Kentucky (2), Louisiana (14), Maryland (6), Massachusetts (56), Michigan (3), Minnesota (7), Missouri (6), Mississippi (2), New Hampshire (2), New Jersey (16), New Mexico (1), New York (32), North Carolina (13), Ohio (3), Oklahoma (1), Oregon (3), Pennsylvania (8), Puerto Rico (3), Rhode Island (2), South Carolina (3), Tennessee (4), Texas (33), Virginia (11), Vermont (1), and Washington (2).
The DVSC25 welcomes 350 underrepresented minorities in medicine and science as our interns represent diverse demographics in gender (691 women & 285 men), age (14~72 years of age with 78% at 18~35 years of age), and ethnicity (173 African American or Black, 6 American Indian or Alaska Native, 50 Hispanic, Latinx or Spanish origin, 121 Middle Eastern or North African, 482 Asian or Asian American, and 93 White).
The DVSC25 interns also represent a diverse status: 104 high school students, 194 college students, 63 post-undergraduates (B.S. or B.A.), 13 nursing students, 29 nutritionists/dieticians, 150 graduate students (Master or Doctoral), 43 pharmacy students, 163 medical students, 27 medical residents (M.D.), 11 clinical fellows (M.D.), 27 postdoctoral researchers (Ph.D. or M.D.), 10 early-career clinicians or research scientists, 56 physicians (M.D. in academic or private), 28 scientists (M.D. or M.D./Ph.D. in academic or industry), 28 faculty (professors), and 38 other health professionals.
Many of our interns have type 1 or type 2 diabetes, or live with families with diabetes. All of our interns share a common goal of advancing knowledge in diabetes and pursuing a career in medicine, research, and other healthcare fields.
Follow us on Facebook (facebook.com/diabetesvirtualcamp), Instagram (instagram.com/diabetesvirtualcamp/), and LinkedIn (linkedin.com/company/diabetesvirtualcamp/).
August 4, 2025
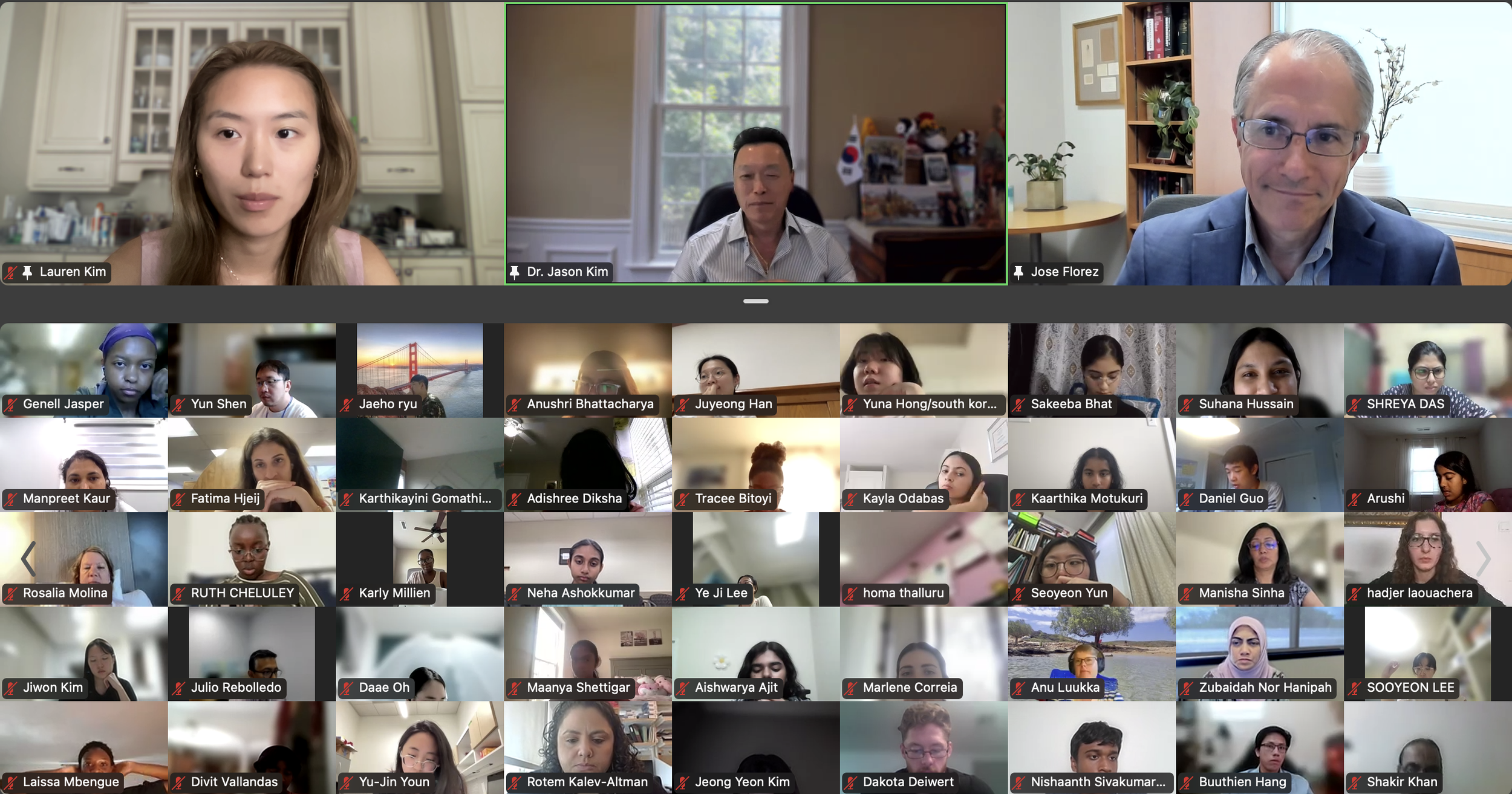
Session 1. Opening Session by DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
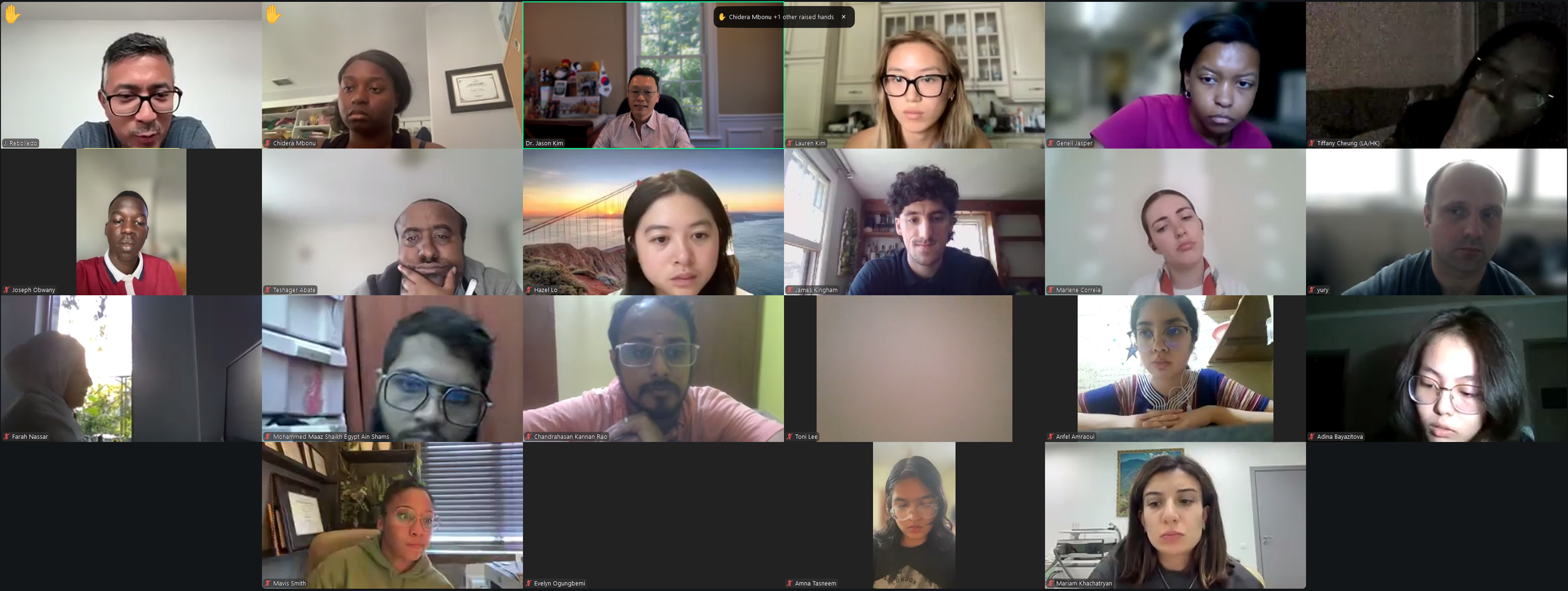
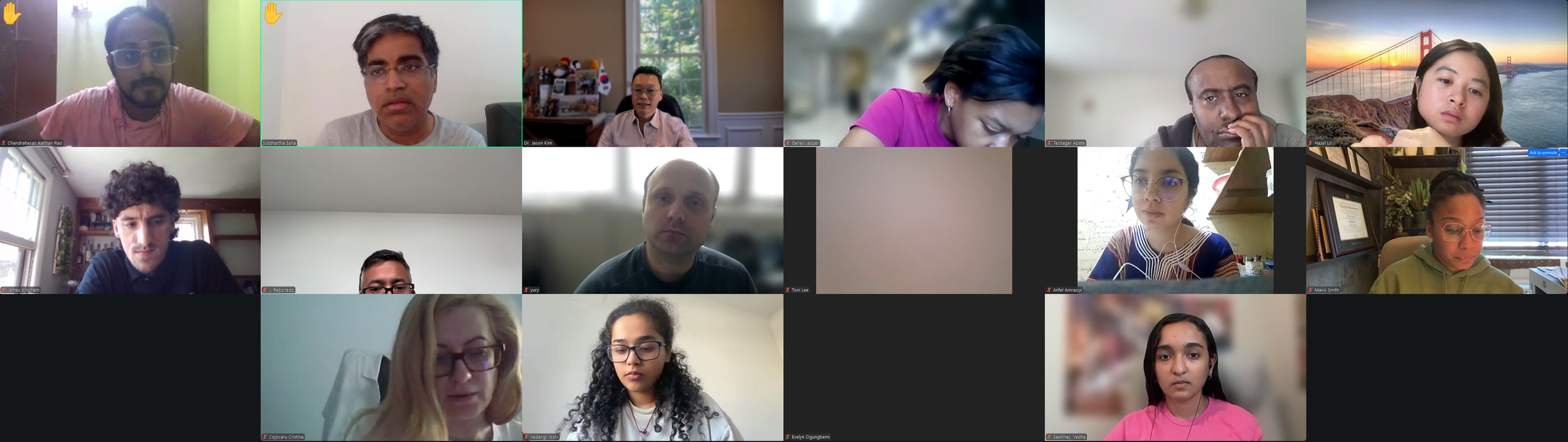
We had an exciting kick-off to Diabetes Virtual Summer Camp 2025 with today’s Opening Session. Lauren Kim (Founder, Program Director, and Webmaster) welcomed the interns from all around the world and shared the story of how this program began. Allison Kim (Associate Director and Director of Outreach) welcomed the interns and discussed the goals of the program and the objectives of the virtual sessions led by leading Experts in the field. Dr. Jason Kim (Advisor) welcomed the interns and discussed the program session format and Zoom policies. Our virtual internship program began in the summer of 2020, at the height of the COVID-19 pandemic, when our world was shut down, and as an effort to use the virtual platform to gather like-minded people, sharing our passion for science, medicine, and research. Since then, our program, now the 8th program of the Diabetes Virtual Camp, has gained new, far-reaching purposes. While scientific and medical conferences serve vital objectives in sharing discoveries, brainstorming with peers, and networking for collaborations, these conferences can be prohibitive to people in many ways, with costly registration fees, expensive travel costs, and even an intimidating environment, none of which applies to our program. Thanks to the American Diabetes Association and the Leona M. and Harry B. Helmsley Charitable Trust, the Diabetes Virtual Camp comes to you, in the comfort of your home or work, no matter where you are, near and far, without any fees, restrictions, or prejudice, as we have gathered today. While COVID-19 took away so many lives and affected other people’s livelihoods, it also changed our society, perhaps some for the better, as we learned that virtual learning has no limits, no boundaries, and no discrimination. When it comes to human health and diseases, understanding and sharing the right information is an important step towards saving lives. That’s why we are here, and that’s the goal of our program. For the next 2 weeks, we will have 13 sessions led by world-renowned clinicians and scientists who will share exciting discoveries from their impactful research, passionate stories about their lifelong care for patients, and inspiring stories of their path to success. We are thrilled to meet interns joining our virtual program from all around the world to better understand diabetes and inspire our next generation of physicians and scientists.
-
![Session 1: Opening Session & Introduction to Diabetes by Lauren, Allison, and Dr. Jason Kim (University of Massachusetts Medical School)]()
August 4, 2025 (Session 1 - Part 1)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Lauren Kim welcomes our program interns and discuss the program goals and objectives.The Diabetes Virtual Summer Camp 2025 celebrates its 5-year anniversary and 8th program with today’s Session 1 - Opening Session where Lauren Kim and Allison Kim welcomed 984 interns, representing over 500 institutions from 70 countries around the world and shared the goals and objectives for the program. The Opening Session ended with many insightful questions from our interns. We are thrilled to meet the interns joining our virtual program from all around the world to better understand diabetes, inspiring our next generation of physicians and scientists.
-
![]()
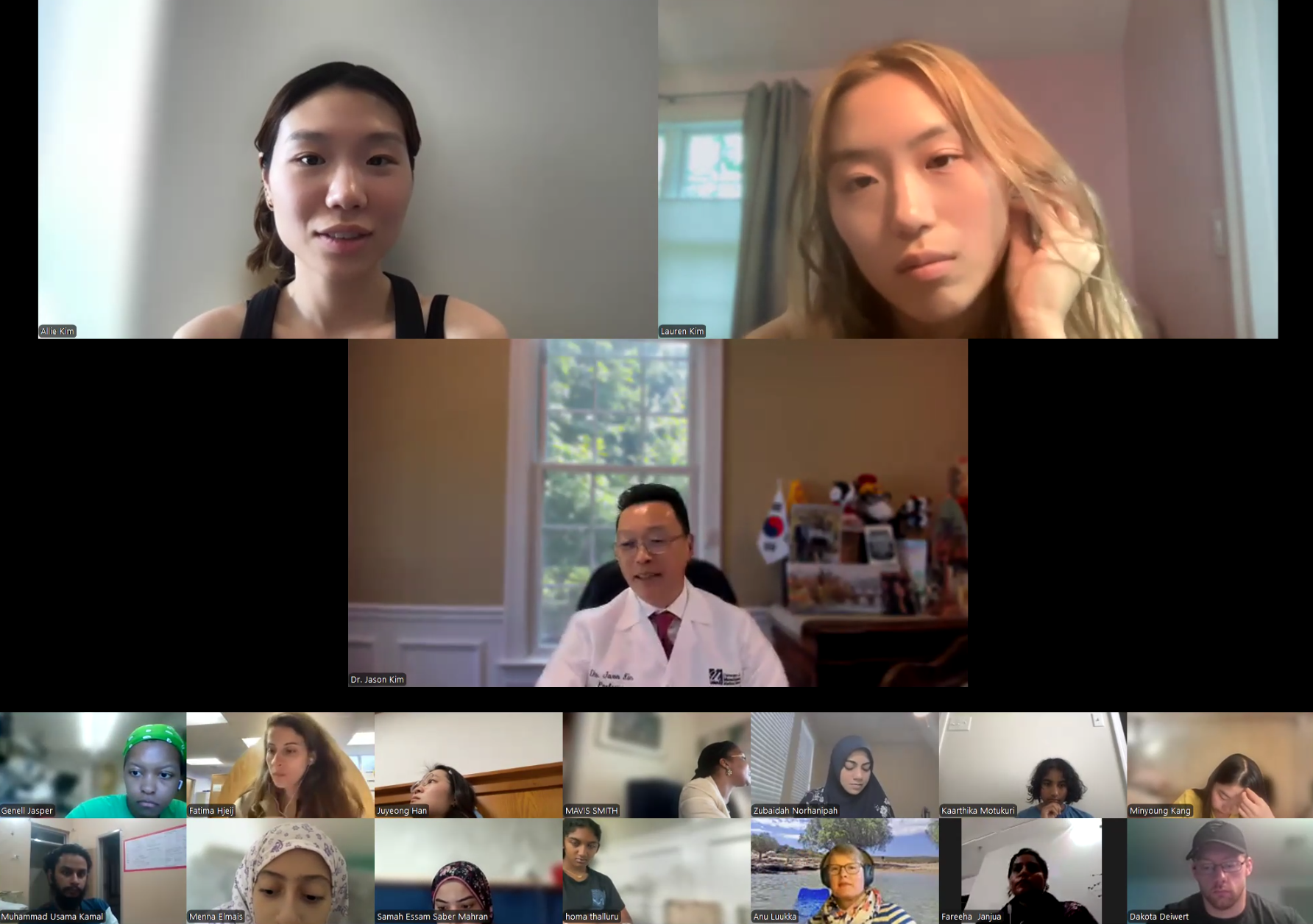
August 4, 2025 (Session 1- Part 2)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Allison Kim welcomes our program interns and discuss the program goals and objectives.The Diabetes Virtual Summer Camp 2025 celebrates its 5-year anniversary and 8th program with today’s Session 1 - Opening Session where Lauren Kim and Allison Kim welcomed 984 interns, representing over 500 institutions from 70 countries around the world and shared the goals and objectives for the program. The Opening Session ended with many insightful questions from our interns. We are thrilled to meet the interns joining our virtual program from all around the world to better understand diabetes, inspiring our next generation of physicians and scientists.
-
![]()
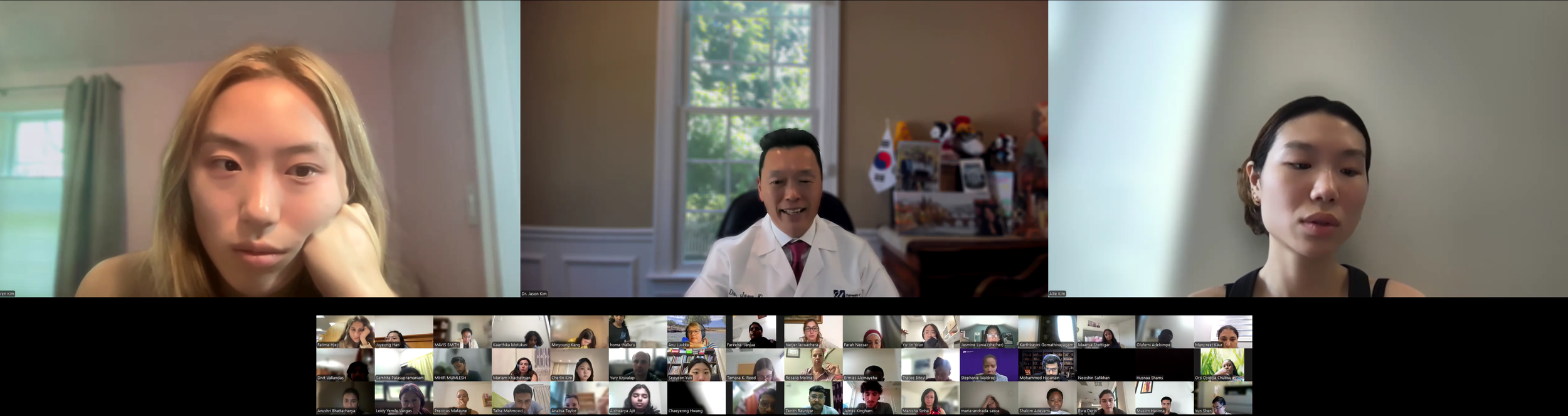
August 4, 2025 (Session 1- Part 3)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Dr. Jason Kim welcomes our program interns and discuss the program goals and objectives.The Diabetes Virtual Summer Camp 2025 celebrates its 5-year anniversary and 8th program with today’s Session 1 - Opening Session where Lauren Kim and Allison Kim welcomed 984 interns, representing over 500 institutions from 70 countries around the world and shared the goals and objectives for the program. The Opening Session ended with many insightful questions from our interns. We are thrilled to meet the interns joining our virtual program from all around the world to better understand diabetes, inspiring our next generation of physicians and scientists.
-
![]()
August 4, 2025 (Session 1- Part 4)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
The DVC Team welcomes our program interns and discuss the program goals and objectives.The Diabetes Virtual Summer Camp 2025 celebrates its 5-year anniversary and 8th program with today’s Session 1 - Opening Session where Lauren Kim and Allison Kim welcomed 984 interns, representing over 500 institutions from 70 countries around the world and shared the goals and objectives for the program. The Opening Session ended with many insightful questions from our interns. We are thrilled to meet the interns joining our virtual program from all around the world to better understand diabetes, inspiring our next generation of physicians and scientists.
-
![]()
August 4, 2025 (Session 1- Part 5)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
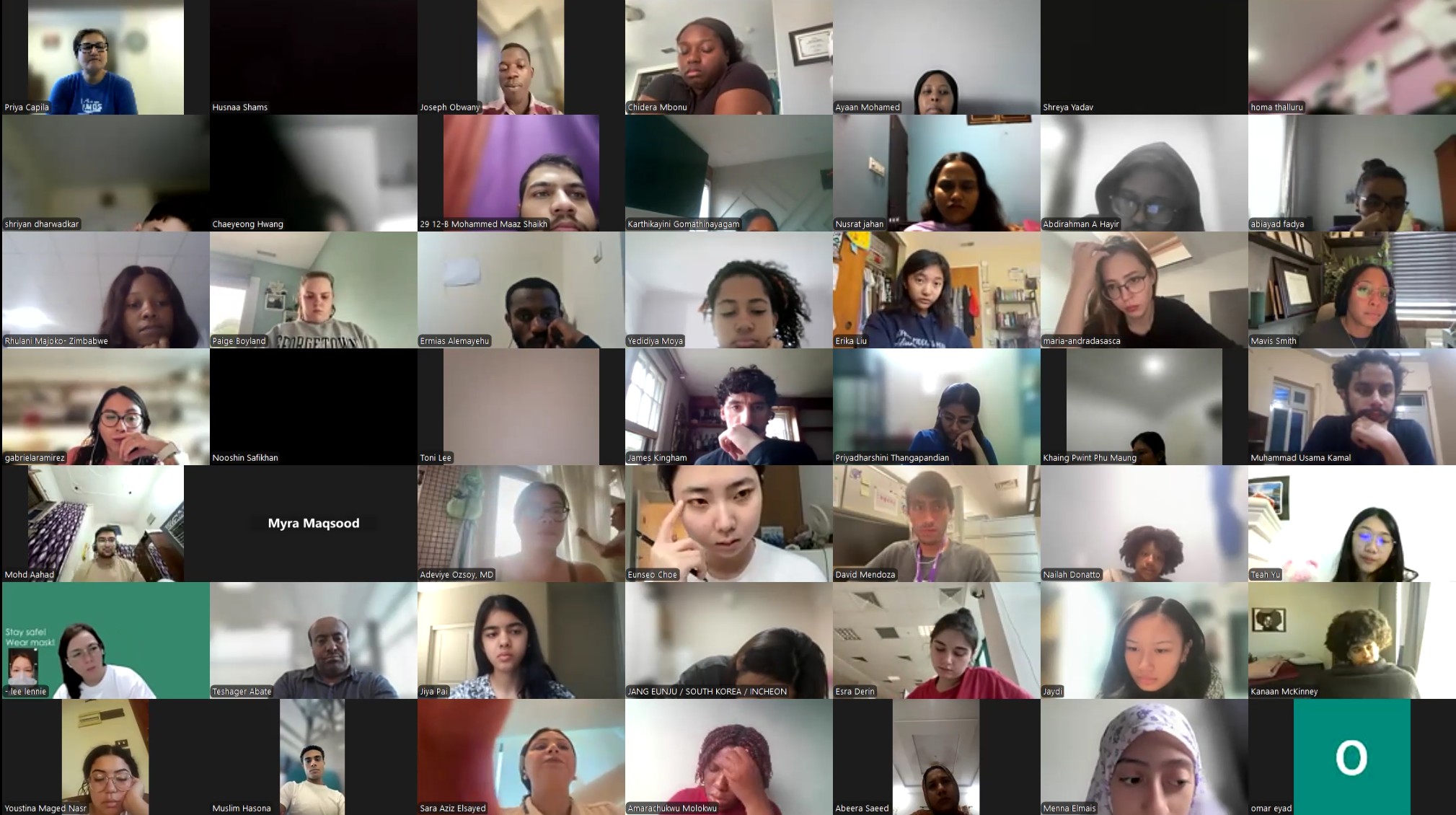
Our DVSC25 interns participate in the Opening Session. Our 984 registered DVSC25 interns represent 70 countries, including Algeria, Armenia, Australia, Bangladesh, Belarus, Belgium, Brazil, Cameroon, Canada, Chile, China, Colombia, Cyprus, Ecuador, Egypt, England, Ethiopia, Finland, France, Georgia, Germany, Ghana, Greece, Hong Kong, Hungary, India, Indonesia, Iraq, Ireland, Italy, Jordan, Kazakhstan, Kenya, Kuwait, Lebanon, Malawi, Malaysia, Mexico, Morocco, Myanmar, Nepal, Netherlands, Nigeria, Pakistan, Palestinian Territories, Philippines, Portugal, Qatar, Romania, Russia, Saudi Arabia, Scotland, Serbia, Singapore, South Korea, Spain, Sri Lanka, Sudan, Sweden, Syria, Tanzania, Thailand, Turkey, Uganda, Ukraine, United Arab Emirates, Vietnam, Zambia, Zimbabwe, and the United States of America. -
![]()
August 4, 2025 (Session 1- Part 6)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Our DVSC25 interns participate in the Opening Session. Our 351 interns from the United States represent 38 states: Alaska (2), Alabama (6), Arkansas (2), Arizona (6), California (53), Colorado (1), Connecticut (4), District of Columbia (1), Florida (5), Georgia (7), Illinois (19), Indiana (9), Kansas (2), Kentucky (2), Louisiana (14), Maryland (6), Massachusetts (56), Michigan (3), Minnesota (7), Missouri (6), Mississippi (2), New Hampshire (2), New Jersey (16), New Mexico (1), New York (32), North Carolina (13), Ohio (3), Oklahoma (1), Oregon (3), Pennsylvania (8), Puerto Rico (3), Rhode Island (2), South Carolina (3), Tennessee (4), Texas (33), Virginia (11), Vermont (1), and Washington (2). -
![]()
August 4, 2025 (Session 1- Part 7)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Our DVSC25 interns participate in the Opening Session. Our 984 registered DVSC25 interns represent diverse demographics in gender (691 women & 285 men), age (14~72 years of age with 78% at 18~35 years of age), and ethnicity (173 African American or Black, 6 American Indian or Alaska Native, 50 Hispanic, Latinx or Spanish origin, 121 Middle Eastern or North African, 482 Asian or Asian American, and 93 White). -
![]()
August 4, 2025 (Session 1- Part 8)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Our DVSC25 interns participate in the Opening Session. Our 984 registered DVSC25 interns represent a diverse current status: 104 high school students, 194 college students, 63 post-undergraduates (B.S. or B.A.), 13 nursing students, 29 nutritionists/dieticians, 150 graduate students (Master or Doctoral), 43 pharmacy students, 163 medical students, 27 medical residents (M.D.), 11 clinical fellows (M.D.), 27 postdoctoral researchers (Ph.D. or M.D.), 10 early-career clinicians or research scientists, 56 physicians (M.D. in academic or private), 28 scientists (Ph.D. or M.D./Ph.D. in academic or industry), 28 faculty (professors), and 38 other health professionals. -
![]()
August 4, 2025 (Session 1- Part 9)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Our DVSC25 interns participate in the Opening Session. Many of our interns have type 1 or type 2 diabetes, or live with families with diabetes. All of our interns share a common goal of advancing their knowledge in diabetes and pursuing a career in medicine, research, and other healthcare fields. -
![]()
August 4, 2025 (Session 1- Part 10)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Our DVSC25 interns participate in the Opening Session. Our 984 registered DVSC25 interns represent 70 countries, including Algeria, Armenia, Australia, Bangladesh, Belarus, Belgium, Brazil, Cameroon, Canada, Chile, China, Colombia, Cyprus, Ecuador, Egypt, England, Ethiopia, Finland, France, Georgia, Germany, Ghana, Greece, Hong Kong, Hungary, India, Indonesia, Iraq, Ireland, Italy, Jordan, Kazakhstan, Kenya, Kuwait, Lebanon, Malawi, Malaysia, Mexico, Morocco, Myanmar, Nepal, Netherlands, Nigeria, Pakistan, Palestinian Territories, Philippines, Portugal, Qatar, Romania, Russia, Saudi Arabia, Scotland, Serbia, Singapore, South Korea, Spain, Sri Lanka, Sudan, Sweden, Syria, Tanzania, Thailand, Turkey, Uganda, Ukraine, United Arab Emirates, Vietnam, Zambia, Zimbabwe, and the United States of America. -
![]()
August 4, 2025 (Session 1- Part 11)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Our DVSC25 interns participate in the Opening Session. Our 351 interns from the United States represent 38 states: Alaska (2), Alabama (6), Arkansas (2), Arizona (6), California (53), Colorado (1), Connecticut (4), District of Columbia (1), Florida (5), Georgia (7), Illinois (19), Indiana (9), Kansas (2), Kentucky (2), Louisiana (14), Maryland (6), Massachusetts (56), Michigan (3), Minnesota (7), Missouri (6), Mississippi (2), New Hampshire (2), New Jersey (16), New Mexico (1), New York (32), North Carolina (13), Ohio (3), Oklahoma (1), Oregon (3), Pennsylvania (8), Puerto Rico (3), Rhode Island (2), South Carolina (3), Tennessee (4), Texas (33), Virginia (11), Vermont (1), and Washington (2). -
![]()
August 4, 2025 (Session 1- Part 12)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Our DVSC25 interns participate in the Opening Session. Our 984 registered DVSC25 interns represent diverse demographics in gender (691 women & 285 men), age (14~72 years of age with 78% at 18~35 years of age), and ethnicity (173 African American or Black, 6 American Indian or Alaska Native, 50 Hispanic, Latinx or Spanish origin, 121 Middle Eastern or North African, 482 Asian or Asian American, and 93 White). -
![]()
August 4, 2025 (Session 1- Part 13)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Our DVSC25 interns participate in the Opening Session. Our 984 registered DVSC25 interns represent a diverse current status: 104 high school students, 194 college students, 63 post-undergraduates (B.S. or B.A.), 13 nursing students, 29 nutritionists/dieticians, 150 graduate students (Master or Doctoral), 43 pharmacy students, 163 medical students, 27 medical residents (M.D.), 11 clinical fellows (M.D.), 27 postdoctoral researchers (Ph.D. or M.D.), 10 early-career clinicians or research scientists, 56 physicians (M.D. in academic or private), 28 scientists (Ph.D. or M.D./Ph.D. in academic or industry), 28 faculty (professors), and 38 other health professionals. -
![]()
August 4, 2025 (Session 1- Part 14)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
Our DVSC25 interns participate in the Opening Session. Many of our interns have type 1 or type 2 diabetes, or live with families with diabetes. All of our interns share a common goal of advancing their knowledge in diabetes and pursuing a career in medicine, research, and other healthcare fields. -
![]()
August 4, 2025 (Session 1- Part 15)
Session 1. Opening Session by DVC Team - Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’), Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’), and Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 4, 2025
Session 2. Introduction to Diabetes by Dr. Jason Kim (University of Massachusetts Chan Medical School)
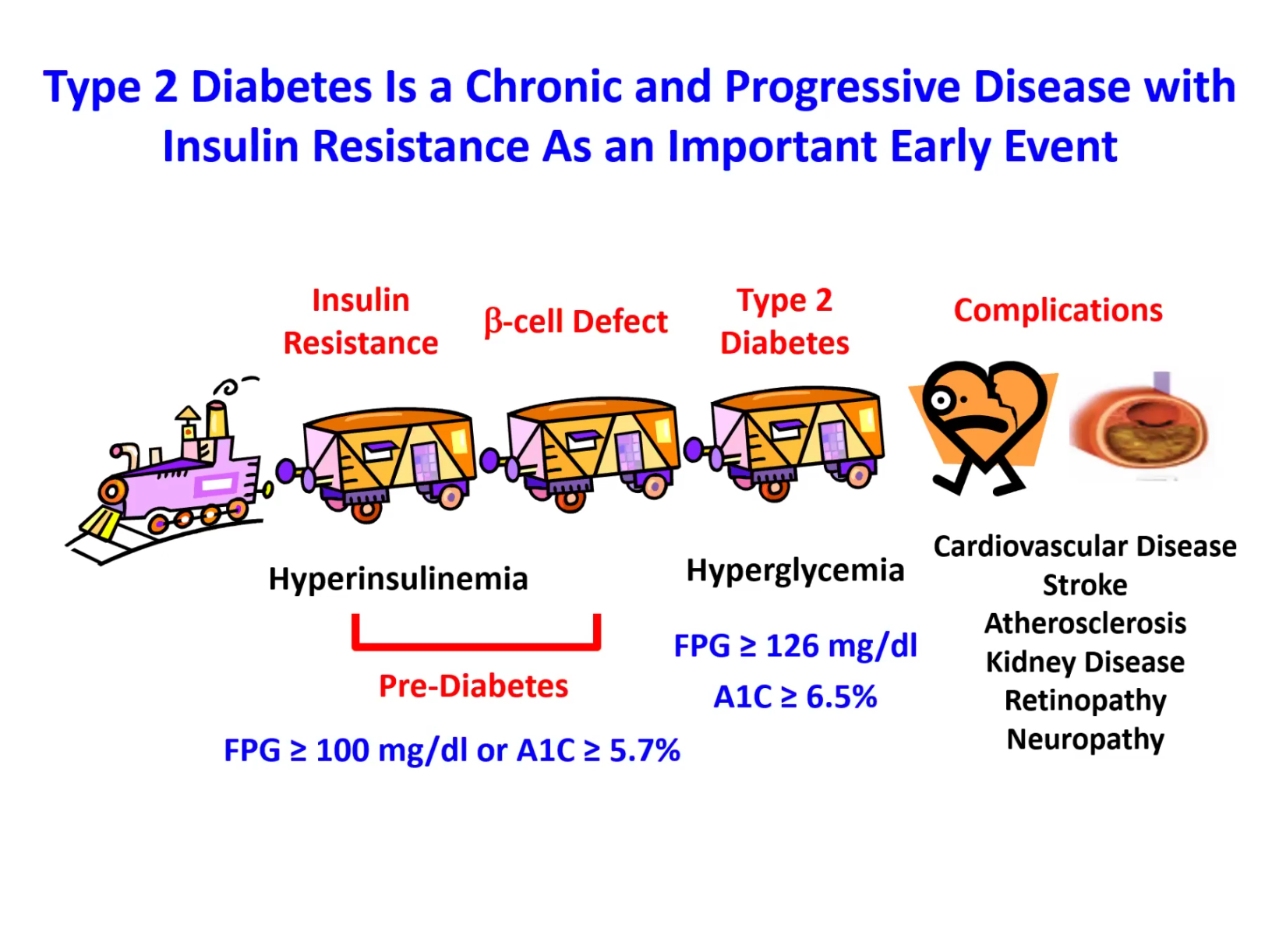
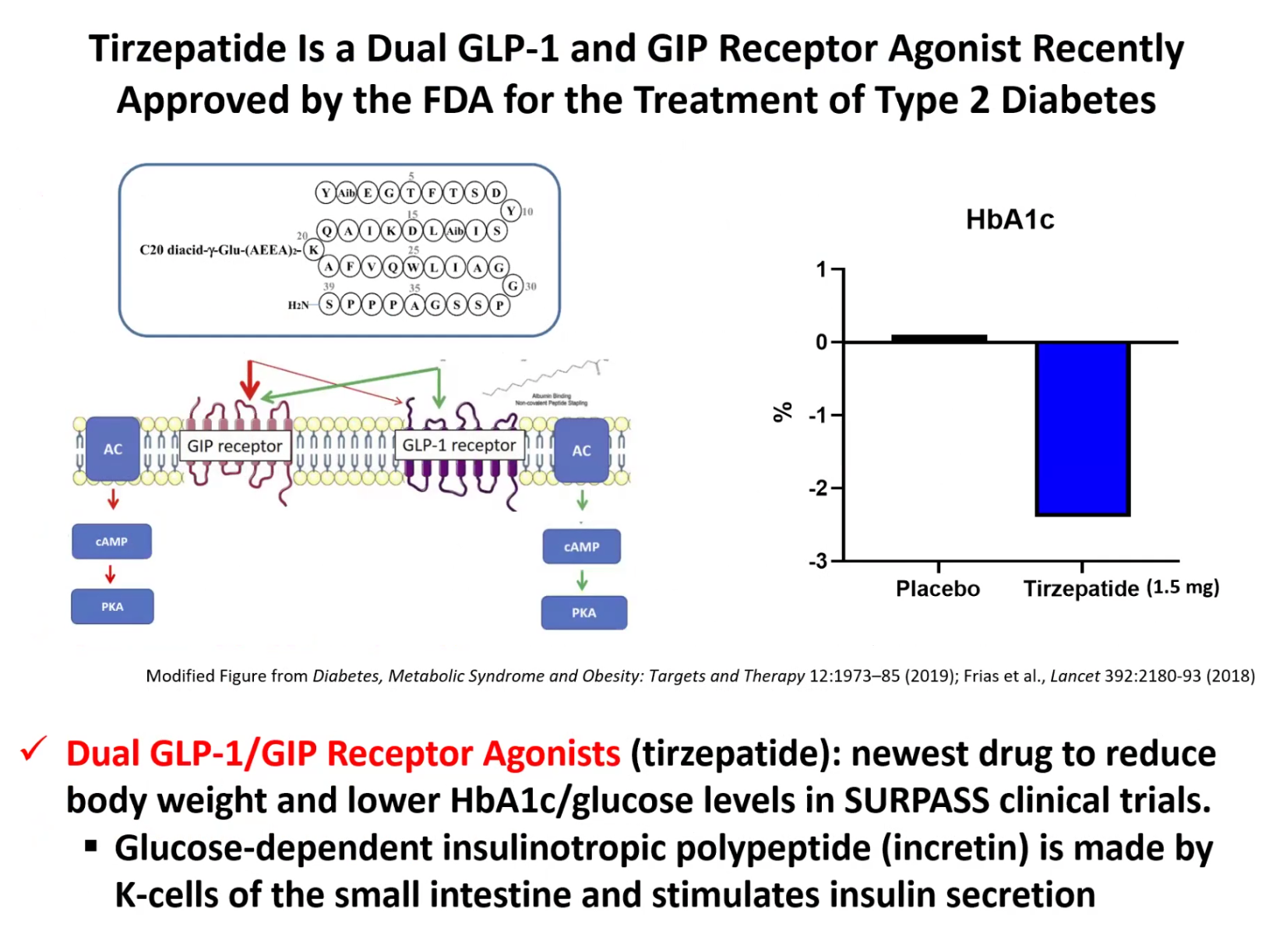
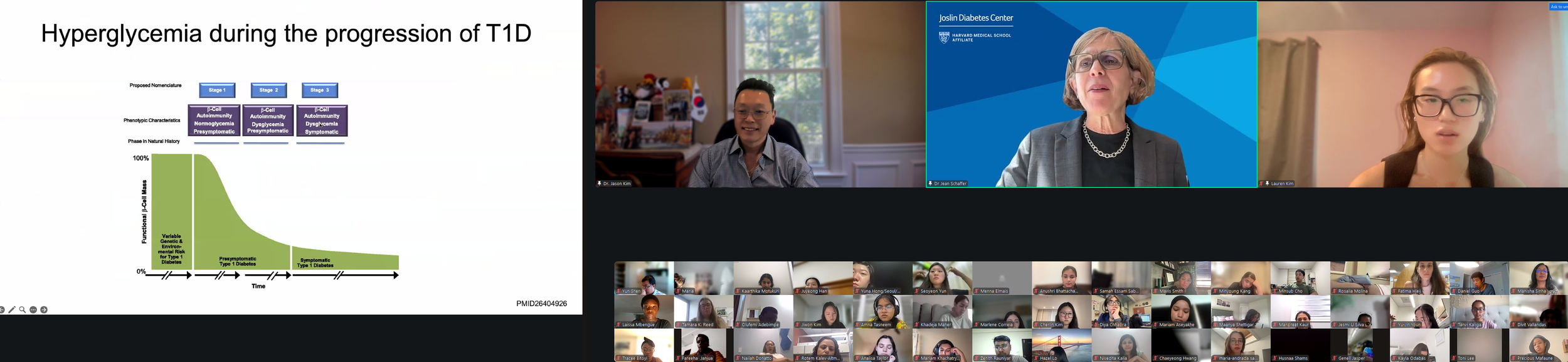
The Expert Session kicked off with “Introduction to Diabetes.” Dr. Jason Kim discussed the basics and pathogenesis of different types of diabetes and their diagnostic criteria, characteristics and etiologies of type 1 and type 2 diabetes, diabetic complications and comorbidities, the importance of glucose homeostasis and how insulin regulates glucose metabolism, insulin action in skeletal muscle and the liver, insulin secretion by pancreatic beta-cells, insulin resistance and pre-diabetes, and progressive events during the development of type 2 diabetes. Dr. Kim also discussed the endocrine role of the pancreas, insulin signaling, molecular pathogenesis of insulin resistance, the role of diets and exercise in preventing and managing diabetes, pharmacological management of diabetes, including the newest class of diabetes drugs, GLP-1 receptor agonists (e.g., semaglutide, exenatide, liraglutide), and our current research on the mechanisms by which tirzepatide regulates insulin sensitivity. Dr. Kim further discussed the role of obesity in type 2 diabetes, multi-factorial causes of obesity and why obesity rates continue to rise globally, different types of fat (white & brown fat), the paradox surrounding lipodystrophy and type 2 diabetes, molecular mechanisms of lipid-mediated insulin resistance, and the role of ectopic fat accumulation in insulin resistance. Dr. Kim ended the session with an insightful question posed by the late Dr. Denis McGarry, “What if Minkowski had been ageusic?”, opening doors to the important notion that while diabetes is manifested as altered carbohydrate metabolism, diabetes is causally associated with altered fat metabolism. The session ended with many insightful questions from our interns. We are thrilled to meet the interns joining our virtual program from all around the world to better understand diabetes, inspiring our next generation of physicians and scientists.
-
![Session 1: Opening Session & Introduction to Diabetes by Lauren, Allison, and Dr. Jason Kim (University of Massachusetts Medical School)]()
August 4, 2025 (Session 2 - Part 1)
Session 2: “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
The Expert Session kicked off with “Introduction to Diabetes.” Dr. Kim discussed the basics and pathogenesis of different types of diabetes and their diagnostic criteria, characteristics and etiologies of type 1 and type 2 diabetes, diabetic complications and comorbidities, the importance of glucose homeostasis and how insulin regulates glucose metabolism, insulin action in skeletal muscle and the liver, insulin secretion by pancreatic beta-cells, insulin resistance and pre-diabetes, and progressive events during the development of type 2 diabetes. Dr. Kim also discussed the endocrine role of the pancreas, insulin signaling, molecular pathogenesis of insulin resistance, the role of diets and exercise in preventing and managing diabetes, pharmacological management of diabetes, including the newest class of diabetes drugs, GLP-1 receptor agonists (e.g., semaglutide, exenatide, liraglutide), and our current research on the mechanisms by which tirzepatide regulates insulin sensitivity. Dr. Kim further discussed the role of obesity in type 2 diabetes, multi-factorial causes of obesity and why obesity rates continue to rise globally, different types of fat (white & brown fat), the paradox surrounding lipodystrophy and type 2 diabetes, molecular mechanisms of lipid-mediated insulin resistance, and the role of ectopic fat accumulation in insulin resistance. Dr. Kim ended the session with an insightful question posed by the late Dr. Denis McGarry, “What if Minkowski had been ageusic?”, opening doors to the important notion that while diabetes is manifested as altered carbohydrate metabolism, diabetes is causally associated with altered fat metabolism. The session ended with many insightful questions from our interns. We are thrilled to meet the interns joining our virtual program from all around the world to better understand diabetes, inspiring our next generation of physicians and scientists. -
![]()
August 4, 2025 (Session 2 - Part 2)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim discussed the basics and pathogenesis of different types of diabetes and their diagnostic criteria, characteristics and etiologies of type 1 and type 2 diabetes, diabetic complications and comorbidities, the importance of glucose homeostasis and how insulin regulates glucose metabolism, insulin action in skeletal muscle and the liver, insulin secretion by pancreatic beta-cells, insulin resistance and pre-diabetes, and progressive events during the development of type 2 diabetes. Dr. Kim also discussed the endocrine role of the pancreas, insulin signaling, molecular pathogenesis of insulin resistance, the role of diets and exercise in preventing and managing diabetes, pharmacological management of diabetes, including the newest class of diabetes drugs, GLP-1 receptor agonists (e.g., semaglutide, exenatide, liraglutide), and our current research on the mechanisms by which tirzepatide regulates insulin sensitivity. Dr. Kim further discussed the role of obesity in type 2 diabetes, multi-factorial causes of obesity and why obesity rates continue to rise globally, different types of fat (white & brown fat), the paradox surrounding lipodystrophy and type 2 diabetes, molecular mechanisms of lipid-mediated insulin resistance, and the role of ectopic fat accumulation in insulin resistance. Dr. Kim ended the session with an insightful question posed by the late Dr. Denis McGarry, “What if Minkowski had been ageusic?”, opening doors to the important notion that while diabetes is manifested as altered carbohydrate metabolism, diabetes is causally associated with altered fat metabolism. The session ended with many insightful questions from our interns. We are thrilled to meet the interns joining our virtual program from all around the world to better understand diabetes, inspiring our next generation of physicians and scientists. -
![]()
August 4, 2025 (Session 2 - Part 3)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School).
Dr. Kim welcomes our program interns from around the world.Our 984 registered DVSC25 interns represent 70 countries, including Algeria, Armenia, Australia, Bangladesh, Belarus, Belgium, Brazil, Cameroon, Canada, Chile, China, Colombia, Cyprus, Ecuador, Egypt, England, Ethiopia, Finland, France, Georgia, Germany, Ghana, Greece, Hong Kong, Hungary, India, Indonesia, Iraq, Ireland, Italy, Jordan, Kazakhstan, Kenya, Kuwait, Lebanon, Malawi, Malaysia, Mexico, Morocco, Myanmar, Nepal, Netherlands, Nigeria, Pakistan, Palestinian Territories, Philippines, Portugal, Qatar, Romania, Russia, Saudi Arabia, Scotland, Serbia, Singapore, South Korea, Spain, Sri Lanka, Sudan, Sweden, Syria, Tanzania, Thailand, Turkey, Uganda, Ukraine, United Arab Emirates, Vietnam, Zambia, Zimbabwe, and the United States of America.
-
![]()
August 4, 2025 (Session 2 - Part 4)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (Professor of Molecular Medicine, Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, and MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
-
![]()
August 4, 2025 (Session 2 - Part 5)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Our 984 registered DVSC25 interns represent 70 countries, including Algeria, Armenia, Australia, Bangladesh, Belarus, Belgium, Brazil, Cameroon, Canada, Chile, China, Colombia, Cyprus, Ecuador, Egypt, England, Ethiopia, Finland, France, Georgia, Germany, Ghana, Greece, Hong Kong, Hungary, India, Indonesia, Iraq, Ireland, Italy, Jordan, Kazakhstan, Kenya, Kuwait, Lebanon, Malawi, Malaysia, Mexico, Morocco, Myanmar, Nepal, Netherlands, Nigeria, Pakistan, Palestinian Territories, Philippines, Portugal, Qatar, Romania, Russia, Saudi Arabia, Scotland, Serbia, Singapore, South Korea, Spain, Sri Lanka, Sudan, Sweden, Syria, Tanzania, Thailand, Turkey, Uganda, Ukraine, United Arab Emirates, Vietnam, Zambia, Zimbabwe, and the United States of America.
-
![]()
August 4, 2025 (Session 2 - Part 6)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Our 351 interns from the United States represent 38 states: Alaska (2), Alabama (6), Arkansas (2), Arizona (6), California (53), Colorado (1), Connecticut (4), District of Columbia (1), Florida (5), Georgia (7), Illinois (19), Indiana (9), Kansas (2), Kentucky (2), Louisiana (14), Maryland (6), Massachusetts (56), Michigan (3), Minnesota (7), Missouri (6), Mississippi (2), New Hampshire (2), New Jersey (16), New Mexico (1), New York (32), North Carolina (13), Ohio (3), Oklahoma (1), Oregon (3), Pennsylvania (8), Puerto Rico (3), Rhode Island (2), South Carolina (3), Tennessee (4), Texas (33), Virginia (11), Vermont (1), and Washington (2).
-
![]()
August 4, 2025 (Session 2 - Part 7)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Our 984 registered DVSC25 interns represent diverse demographics in gender (691 women & 285 men), age (14~72 years of age with 78% at 18~35 years of age), and ethnicity (173 African American or Black, 6 American Indian or Alaska Native, 50 Hispanic, Latinx or Spanish origin, 121 Middle Eastern or North African, 482 Asian or Asian American, and 93 White).
-
![]()
August 4, 2025 (Session 2 - Part 8)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Our 984 registered DVSC25 interns represent a diverse current status: 104 high school students, 194 college students, 63 post-undergraduates (B.S. or B.A.), 13 nursing students, 29 nutritionists/dieticians, 150 graduate students (Master or Doctoral), 43 pharmacy students, 163 medical students, 27 medical residents (M.D.), 11 clinical fellows (M.D.), 27 postdoctoral researchers (Ph.D. or M.D.), 10 early-career clinicians or research scientists, 56 physicians (M.D. in academic or private), 28 scientists (Ph.D. or M.D./Ph.D. in academic or industry), 28 faculty (professors), and 38 other health professionals.
-
![]()
August 4, 2025 (Session 2 - Part 9)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Many of our interns have type 1 or type 2 diabetes, or live with families with diabetes. All of our interns share a common goal of advancing their knowledge in diabetes and pursuing a career in medicine, research, and other healthcare fields.
-
![]()
August 4, 2025 (Session 2 - Part 10)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Dr. Jason Kim discussed the basics and pathogenesis of different types of diabetes and their diagnostic criteria, characteristics and etiologies of type 1 and type 2 diabetes, diabetic complications and comorbidities, the importance of glucose homeostasis and how insulin regulates glucose metabolism, insulin action in skeletal muscle and the liver, insulin secretion by pancreatic beta-cells, insulin resistance and pre-diabetes, and progressive events during the development of type 2 diabetes.
-
![]()
August 4, 2025 (Session 2 - Part 11)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Dr. Kim discussed the endocrine role of the pancreas, insulin signaling, molecular pathogenesis of insulin resistance, the role of diets and exercise in preventing and managing diabetes, pharmacological management of diabetes, including the newest class of diabetes drugs, GLP-1 receptor agonists (e.g., semaglutide, exenatide, liraglutide), and our current research on the mechanisms by which tirzepatide regulates insulin sensitivity.
-
![]()
August 4, 2025 (Session 2 - Part 12)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Dr. Kim further discussed the role of obesity in type 2 diabetes, multi-factorial causes of obesity and why obesity rates continue to rise globally, different types of fat (white & brown fat), the paradox surrounding lipodystrophy and type 2 diabetes, molecular mechanisms of lipid-mediated insulin resistance, and the role of ectopic fat accumulation in insulin resistance.
-
![]()
August 4, 2025 (Session 2 - Part 13)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Dr. Kim discussed pharmacological management of diabetes, including the newest class of diabetes drugs, GLP-1 receptor agonists (e.g., semaglutide, exenatide, liraglutide), and our current research on the mechanisms by which tirzepatide regulates insulin sensitivity.
-
![]()
August 4, 2025 (Session 2 - Part 14)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.This session introduced diabetes: different types and diagnostic criteria of diabetes, characteristics and etiologies of type 1 and type 2 diabetes, diabetic complications and comorbidities, the importance of glucose homeostasis and how insulin regulates glucose metabolism, insulin signaling and insulin secretion by the beta-cells, insulin resistance and pre-diabetes, and progressive events during the development of type 2 diabetes. The session further discussed how to prevent and manage type 2 diabetes using lifestyle modifications (diets and exercise) and different drugs for treating diabetes.
-
![]()
August 4, 2025 (Session 2 - Part 15)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Dr. Kim discussed the endocrine role of the pancreas, insulin signaling, molecular pathogenesis of insulin resistance, the role of diets and exercise in preventing and managing diabetes, pharmacological management of diabetes, including the newest class of diabetes drugs, GLP-1 receptor agonists (e.g., semaglutide, exenatide, liraglutide), and our current research on the mechanisms by which tirzepatide regulates insulin sensitivity.
-
![]()
August 4, 2025 (Session 2 - Part 16)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Dr. Kim further discussed the role of obesity in type 2 diabetes, multi-factorial causes of obesity and why obesity rates continue to rise globally, different types of fat (white & brown fat), the paradox surrounding lipodystrophy and type 2 diabetes, molecular mechanisms of lipid-mediated insulin resistance, and the role of ectopic fat accumulation in insulin resistance.
-
![]()
August 4, 2025 (Session 2 - Part 17)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.This session introduced diabetes: different types and diagnostic criteria of diabetes, characteristics and etiologies of type 1 and type 2 diabetes, diabetic complications and comorbidities, the importance of glucose homeostasis and how insulin regulates glucose metabolism, insulin signaling and insulin secretion by the beta-cells, insulin resistance and pre-diabetes, and progressive events during the development of type 2 diabetes. The session further discussed how to prevent and manage type 2 diabetes using lifestyle modifications (diets and exercise) and different drugs for treating diabetes.
-
![]()
August 4, 2025 (Session 2 - Part 18)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Our DVSC25 interns learn about the basics of diabetes.Dr. Kim ended the session with an insightful question posed by the late Dr. Denis McGarry, “What if Minkowski had been ageusic?”, opening doors to the important notion that while diabetes is manifested as altered carbohydrate metabolism, diabetes is causally associated with altered fat metabolism.
-
![]()
August 4, 2025 (Session 2 - Part 19)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim expresses his gratitude to the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute on Aging for their vital support of his research program, advancing biological understanding of type 2 diabetes, obesity, fatty liver disease, and Alzheimer’s disease, and ultimately saving people’s lives. -
![]()
August 4, 2025 (Session 2 - Part 20)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim acknowledges the passion, efforts, and dedication of his research team at the University of Massachusetts Chan Medical School as they work together to cure diabetes and its comorbidities. -
![]()
August 4, 2025 (Session 2 - Part 21)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School).
Dr. Kim invites our interns to ask questions. -
![]()
August 4, 2025 (Session 2 - Part 22)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School).
Dr. Kim invites our interns to ask questions. -
![]()
August 4, 2025 (Session 2 - Part 23)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School).
Dr. Kim invites our interns to ask questions. -
![]()
August 4, 2025 (Session 2 - Part 24)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School).
Dr. Kim invites our interns to ask questions. -
![]()
August 4, 2025 (Session 2 - Part 25)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School).
Dr. Kim addresses thoughtful questions from our interns. -
![]()
August 4, 2025 (Session 2 - Part 26)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School).
Dr. Kim addresses thoughtful questions from our interns. -
![]()
August 4, 2025 (Session 2 - Part 27)
Session 1-2. Open Mic Forum by the DVC Team.
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 4, 2025 (Session 2 - Part 28)
Session 1-2. Open Mic Forum by the DVC Team.
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 4, 2025 (Session 2 - Part 29)
Session 2. “Introduction to Diabetes” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 6, 2025
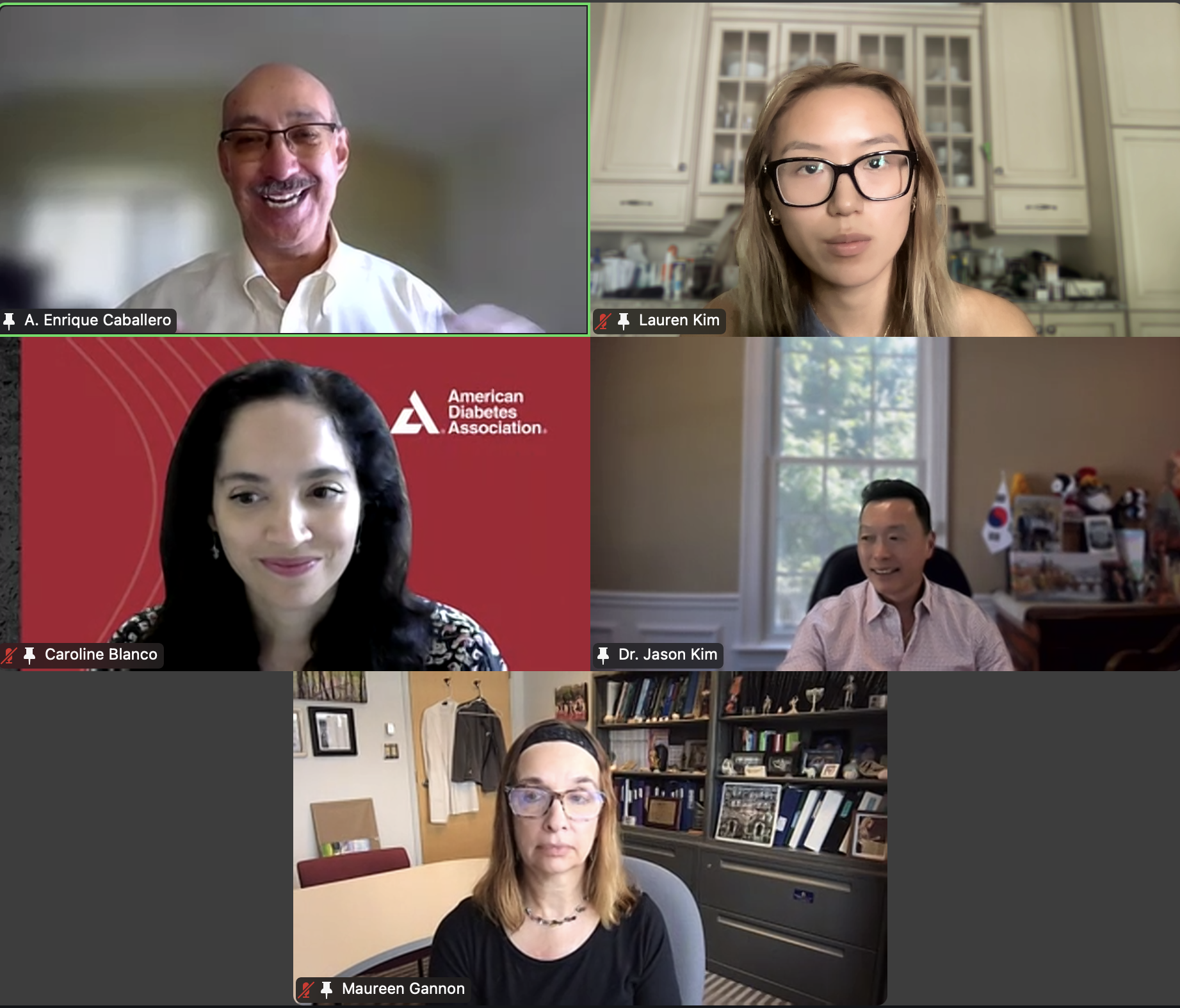
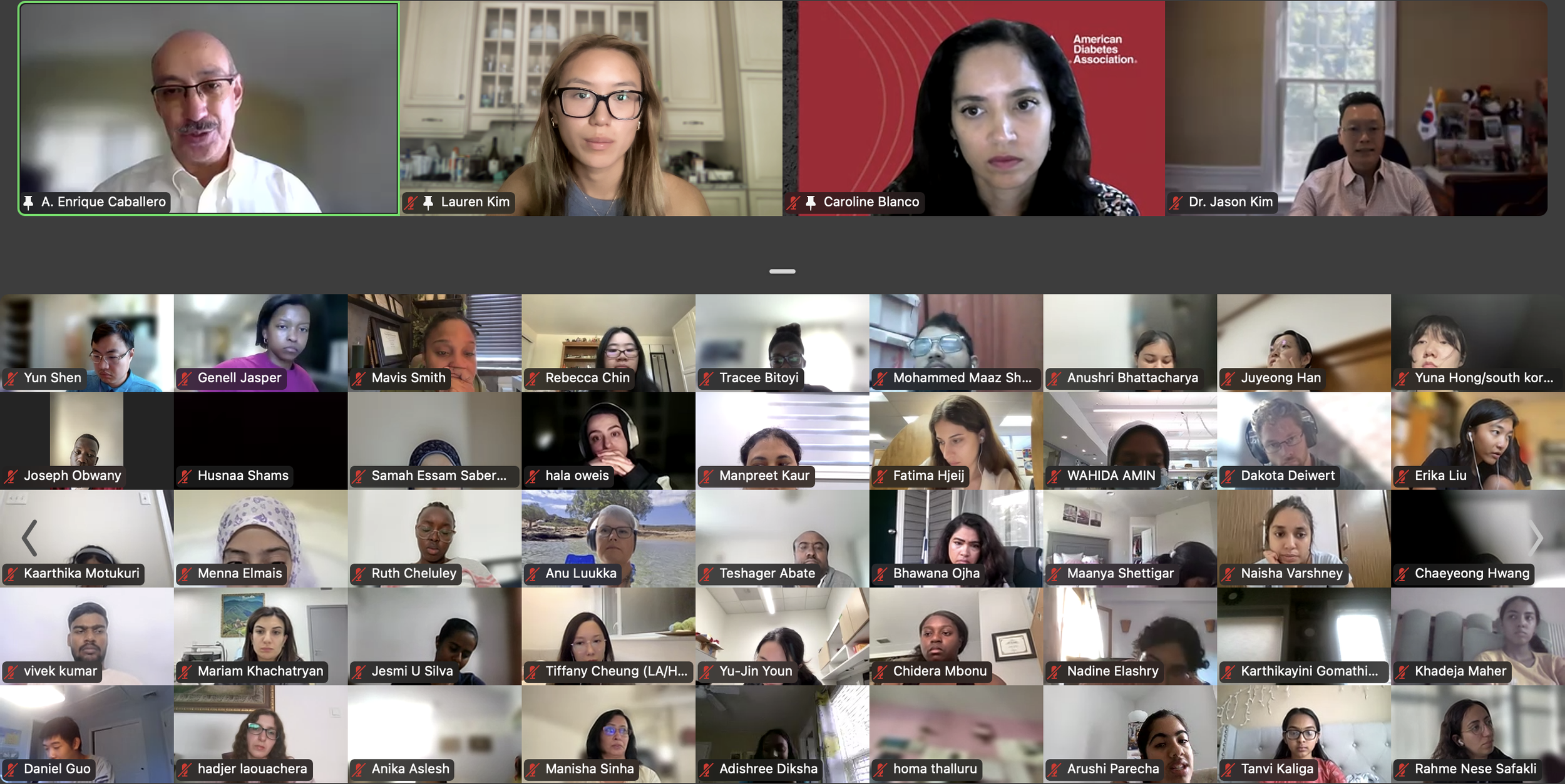
Session 3. Why You Shouldn’t Drink Sweet Things by Dr. Richard Lee (Harvard University)
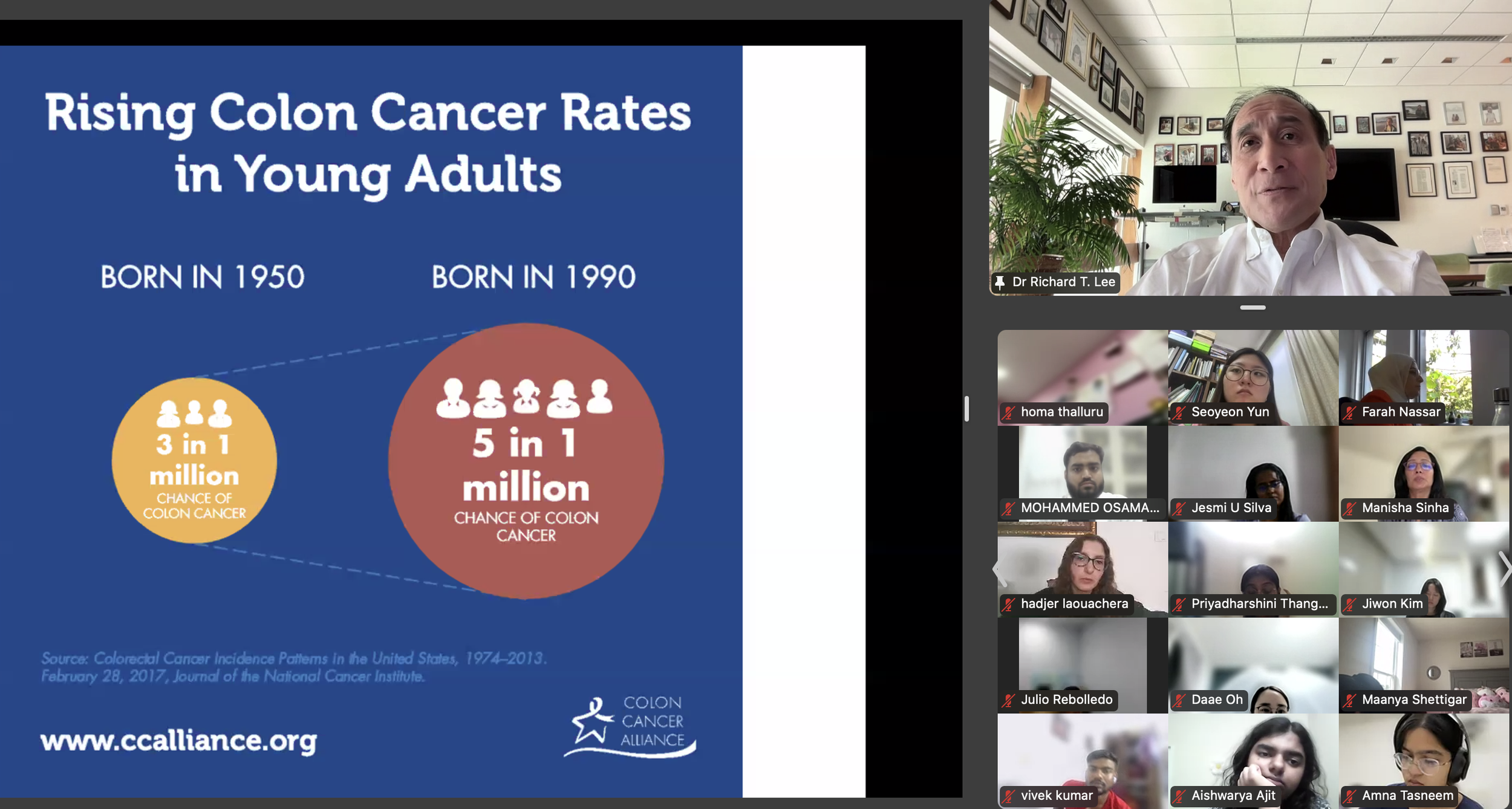
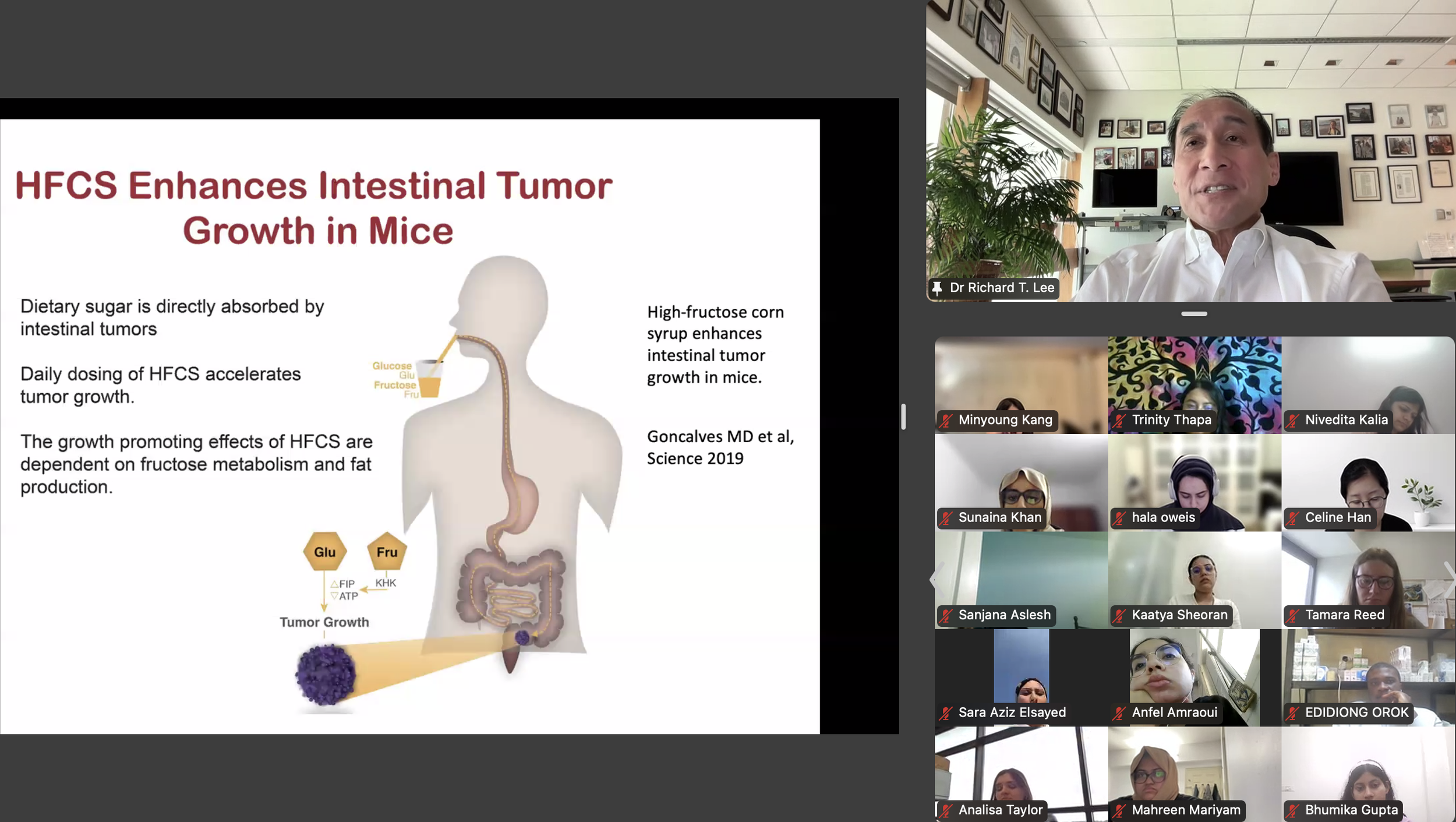
The DVSC25 continued today with 2 incredible Experts, Dr. Richard Lee from Harvard University and Dr. Jose Florez from Harvard Medical School. Dr. Lee began by highlighting a disturbing trend of increasing fructose consumption in the U.S., particularly in liquid form, and this trend is associated with rising rates of metabolic diseases. This undesirable relationship is exacerbated by the addition of high fructose corn syrup (HFCS), an inexpensive and more common source of dietary sugar. Dr. Lee further discussed how fructose is metabolized by the intestine and liver and contributes to fatty liver disease. The HFCS has also been shown to enhance intestinal tumor growth in mice, possibly relating to the rising colon cancer rates in young adults with high consumption of liquid fructose. While sweetened beverages are shown to increase mortality, diet sodas are also associated with an increased risk of cardiovascular disease. Large liquid phase fructose loads may be worse because lower or slowly absorbed doses are converted to glucose in the intestine, likely a natural protective mechanism that we overwhelm with sugary drinks (evolutionary mismatch!). Bottom line, Dr. Lee recommends that it’s better to eat an orange than to drink orange juice. The session ended with many insightful questions from our interns.
-
![]()
August 6, 2025 (Session 3 - Part 1)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
The DVSC25 continued today with 2 incredible Experts, Dr. Richard Lee from Harvard University and Dr. Jose Florez from Harvard Medical School. -
![]()
August 6, 2025 (Session 3 - Part 2)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
The DVC Team introduces Dr. Richard Lee to our Interns from all around the world. -
![]()
August 6, 2025 (Session 3 - Part 3)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
This session discusses fructose as an important sugar in our daily lives, and how fructose can influence glucose metabolism. We will address basic mechanisms of fructose metabolism, and how fructose is similar to glucose but very different in some settings. -
![]()
August 6, 2025 (Session 3 - Part 4)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Lee welcomes our program Interns from around the world. -
![]()
August 6, 2025 (Session 3 - Part 5)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Lee welcomes our program interns from around the world. -
![]()
August 6, 2025 (Session 3 - Part 6)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Lee welcomes our program interns from around the world. -
![]()
August 6, 2025 (Session 3 - Part 7)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Lee welcomes our program interns from around the world. -
![]()
August 6, 2025 (Session 3 - Part 8)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Professor of Stem Cell and Regenerative Biology, Harvard Stem Cell Institute, Harvard University and Professor of Medicine, Brigham & Women’s Hospital, Harvard Medical School)
-
![]()
August 6, 2025 (Session 3 - Part 9)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Richard Lee began by highlighting a disturbing trend of increasing fructose consumption in the U.S., particularly in liquid form, and this trend is associated with rising rates of metabolic diseases. -
![]()
August 6, 2025 (Session 3 - Part 10)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Richard Lee began by highlighting a disturbing trend of increasing fructose consumption in the U.S., particularly in liquid form, and this trend is associated with rising rates of metabolic diseases. This undesirable relationship is exacerbated by the addition of high fructose corn syrup (HFCS), an inexpensive and more common source of dietary sugar. Dr. Lee further discussed how fructose is metabolized by the intestine and liver and contributes to fatty liver disease. The HFCS has also been shown to enhance intestinal tumor growth in mice, possibly relating to the rising colon cancer rates in young adults with high consumption of liquid fructose. While sweetened beverages are shown to increase mortality, diet sodas are also associated with an increased risk of cardiovascular disease. Large liquid phase fructose loads may be worse because lower or slowly absorbed doses are converted to glucose in the intestine, likely a natural protective mechanism that we overwhelm with sugary drinks (evolutionary mismatch!). Bottom line, Dr. Lee recommends that it’s better to eat an orange than to drink orange juice. -
![]()
August 6, 2025 (Session 3 - Part 11)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Richard Lee began by highlighting a disturbing trend of increasing fructose consumption in the U.S., particularly in liquid form, and this trend is associated with rising rates of metabolic diseases. This undesirable relationship is exacerbated by the addition of high fructose corn syrup (HFCS), an inexpensive and more common source of dietary sugar. Dr. Lee further discussed how fructose is metabolized by the intestine and liver and contributes to fatty liver disease. The HFCS has also been shown to enhance intestinal tumor growth in mice, possibly relating to the rising colon cancer rates in young adults with high consumption of liquid fructose. While sweetened beverages are shown to increase mortality, diet sodas are also associated with an increased risk of cardiovascular disease. Large liquid phase fructose loads may be worse because lower or slowly absorbed doses are converted to glucose in the intestine, likely a natural protective mechanism that we overwhelm with sugary drinks (evolutionary mismatch!). Bottom line, Dr. Lee recommends that it’s better to eat an orange than to drink orange juice. -
![]()
August 6, 2025 (Session 3 - Part 12)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Richard Lee began by highlighting a disturbing trend of increasing fructose consumption in the U.S., particularly in liquid form, and this trend is associated with rising rates of metabolic diseases. This undesirable relationship is exacerbated by the addition of high fructose corn syrup (HFCS), an inexpensive and more common source of dietary sugar. -
![]()
August 6, 2025 (Session 3 - Part 13)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
This session discusses fructose as an important sugar in our daily lives, and how fructose can influence glucose metabolism. We will address basic mechanisms of fructose metabolism, and how fructose is similar to glucose but very different in some settings. -
![]()
August 6, 2025 (Session 3 - Part 14)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
This session discusses fructose as an important sugar in our daily lives, and how fructose can influence glucose metabolism. We will address basic mechanisms of fructose metabolism, and how fructose is similar to glucose but very different in some settings. -
![]()
August 6, 2025 (Session 3 - Part 15)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Lee further discussed how fructose is metabolized by the intestine and liver and contributes to fatty liver disease. The HFCS has also been shown to enhance intestinal tumor growth in mice, possibly relating to the rising colon cancer rates in young adults with high consumption of liquid fructose. While sweetened beverages are shown to increase mortality, diet sodas are also associated with an increased risk of cardiovascular disease. Large liquid phase fructose loads may be worse because lower or slowly absorbed doses are converted to glucose in the intestine, likely a natural protective mechanism that we overwhelm with sugary drinks (evolutionary mismatch!). Bottom line, Dr. Lee recommends that it’s better to eat an orange than to drink orange juice. -
![]()
August 6, 2025 (Session 3 - Part 16)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Richard Lee began by highlighting a disturbing trend of increasing fructose consumption in the U.S., particularly in liquid form, and this trend is associated with rising rates of metabolic diseases. This undesirable relationship is exacerbated by the addition of high fructose corn syrup (HFCS), an inexpensive and more common source of dietary sugar. Dr. Lee further discussed how fructose is metabolized by the intestine and liver and contributes to fatty liver disease. The HFCS has also been shown to enhance intestinal tumor growth in mice, possibly relating to the rising colon cancer rates in young adults with high consumption of liquid fructose. While sweetened beverages are shown to increase mortality, diet sodas are also associated with an increased risk of cardiovascular disease. Large liquid phase fructose loads may be worse because lower or slowly absorbed doses are converted to glucose in the intestine, likely a natural protective mechanism that we overwhelm with sugary drinks (evolutionary mismatch!). Bottom line, Dr. Lee recommends that it’s better to eat an orange than to drink orange juice. -
![]()
August 6, 2025 (Session 3 - Part 17)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Richard Lee began by highlighting a disturbing trend of increasing fructose consumption in the U.S., particularly in liquid form, and this trend is associated with rising rates of metabolic diseases. This undesirable relationship is exacerbated by the addition of high fructose corn syrup (HFCS), an inexpensive and more common source of dietary sugar. Dr. Lee further discussed how fructose is metabolized by the intestine and liver and contributes to fatty liver disease. The HFCS has also been shown to enhance intestinal tumor growth in mice, possibly relating to the rising colon cancer rates in young adults with high consumption of liquid fructose. While sweetened beverages are shown to increase mortality, diet sodas are also associated with an increased risk of cardiovascular disease. Large liquid phase fructose loads may be worse because lower or slowly absorbed doses are converted to glucose in the intestine, likely a natural protective mechanism that we overwhelm with sugary drinks (evolutionary mismatch!). Bottom line, Dr. Lee recommends that it’s better to eat an orange than to drink orange juice. -
![]()
August 6, 2025 (Session 3 - Part 18)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Lee further discussed how fructose is metabolized by the intestine and liver and contributes to fatty liver disease. The HFCS has also been shown to enhance intestinal tumor growth in mice, possibly relating to the rising colon cancer rates in young adults with high consumption of liquid fructose. While sweetened beverages are shown to increase mortality, diet sodas are also associated with an increased risk of cardiovascular disease. Large liquid phase fructose loads may be worse because lower or slowly absorbed doses are converted to glucose in the intestine, likely a natural protective mechanism that we overwhelm with sugary drinks (evolutionary mismatch!). Bottom line, Dr. Lee recommends that it’s better to eat an orange than to drink orange juice. -
![]()
August 6, 2025 (Session 3 - Part 19)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Richard Lee began by highlighting a disturbing trend of increasing fructose consumption in the U.S., particularly in liquid form, and this trend is associated with rising rates of metabolic diseases. This undesirable relationship is exacerbated by the addition of high fructose corn syrup (HFCS), an inexpensive and more common source of dietary sugar. Dr. Lee further discussed how fructose is metabolized by the intestine and liver and contributes to fatty liver disease. The HFCS has also been shown to enhance intestinal tumor growth in mice, possibly relating to the rising colon cancer rates in young adults with high consumption of liquid fructose. While sweetened beverages are shown to increase mortality, diet sodas are also associated with an increased risk of cardiovascular disease. Large liquid phase fructose loads may be worse because lower or slowly absorbed doses are converted to glucose in the intestine, likely a natural protective mechanism that we overwhelm with sugary drinks (evolutionary mismatch!). Bottom line, Dr. Lee recommends that it’s better to eat an orange than to drink orange juice. -
![]()
August 6, 2025 (Session 3 - Part 20)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
This session discusses fructose as an important sugar in our daily lives, and how fructose can influence glucose metabolism. We will address basic mechanisms of fructose metabolism, and how fructose is similar to glucose but very different in some settings. -
![]()
August 6, 2025 (Session 3 - Part 21)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Richard Lee began by highlighting a disturbing trend of increasing fructose consumption in the U.S., particularly in liquid form, and this trend is associated with rising rates of metabolic diseases. This undesirable relationship is exacerbated by the addition of high fructose corn syrup (HFCS), an inexpensive and more common source of dietary sugar. Dr. Lee further discussed how fructose is metabolized by the intestine and liver and contributes to fatty liver disease. The HFCS has also been shown to enhance intestinal tumor growth in mice, possibly relating to the rising colon cancer rates in young adults with high consumption of liquid fructose. While sweetened beverages are shown to increase mortality, diet sodas are also associated with an increased risk of cardiovascular disease. Large liquid phase fructose loads may be worse because lower or slowly absorbed doses are converted to glucose in the intestine, likely a natural protective mechanism that we overwhelm with sugary drinks (evolutionary mismatch!). Bottom line, Dr. Lee recommends that it’s better to eat an orange than to drink orange juice. -
![]()
August 6, 2025 (Session 3 - Part 22)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
This session discusses fructose as an important sugar in our daily lives, and how fructose can influence glucose metabolism. We will address basic mechanisms of fructose metabolism, and how fructose is similar to glucose but very different in some settings. -
![]()
August 6, 2025 (Session 3 - Part 23)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
This session discusses fructose as an important sugar in our daily lives, and how fructose can influence glucose metabolism. We will address basic mechanisms of fructose metabolism, and how fructose is similar to glucose but very different in some settings. -
![]()
August 6, 2025 (Session 3 - Part 24)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Richard Lee began by highlighting a disturbing trend of increasing fructose consumption in the U.S., particularly in liquid form, and this trend is associated with rising rates of metabolic diseases. This undesirable relationship is exacerbated by the addition of high fructose corn syrup (HFCS), an inexpensive and more common source of dietary sugar. Dr. Lee further discussed how fructose is metabolized by the intestine and liver and contributes to fatty liver disease. The HFCS has also been shown to enhance intestinal tumor growth in mice, possibly relating to the rising colon cancer rates in young adults with high consumption of liquid fructose. While sweetened beverages are shown to increase mortality, diet sodas are also associated with an increased risk of cardiovascular disease. Large liquid phase fructose loads may be worse because lower or slowly absorbed doses are converted to glucose in the intestine, likely a natural protective mechanism that we overwhelm with sugary drinks (evolutionary mismatch!). Bottom line, Dr. Lee recommends that it’s better to eat an orange than to drink orange juice. -
![]()
August 6, 2025 (Session 3 - Part 25)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Lee invites our interns to ask questions. -
![]()
August 6, 2025 (Session 3 - Part 26)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Lauren moderates the Q&A period as Dr. Lee addresses thoughtful questions from our interns. -
![]()
August 6, 2025 (Session 3 - Part 27)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Lauren moderates the Q&A period as Dr. Lee addresses thoughtful questions from our interns. -
![]()
August 6, 2025 (Session 3 - Part 28)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Dr. Lee addresses thoughtful questions from our interns. -
![]()
August 6, 2025 (Session 3 - Part 29)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
The DVC Team and our Interns thank Dr. Lee for a fantastic presentation. -
![]()
August 6, 2025 (Session 3 - Part 30)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 6, 2025
Session 4. Precision Medicine in Diabetes by Dr. Jose Florez (Harvard Medical School)
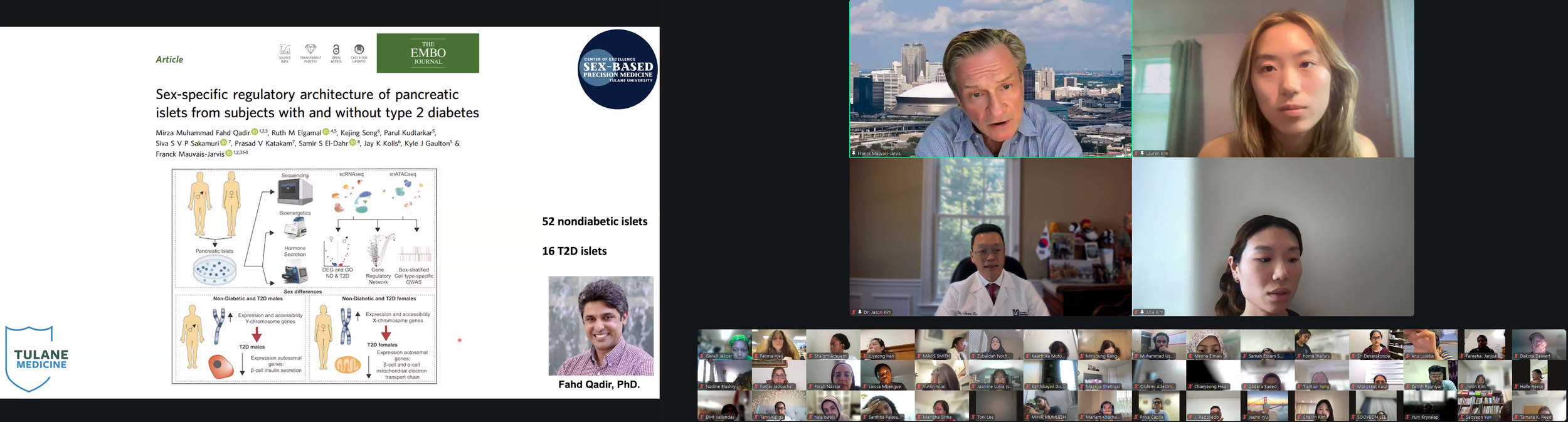
The DVSC25 continued today with 2 incredible Experts, Dr. Richard Lee from Harvard University and Dr. Jose Florez from Harvard Medical School. Today’s exciting program continued with Dr. Jose Florez, who began by highlighting the heterogeneity of diabetes and an important finding from the Leif group showing how diabetic subjects could be clustered based on different clinical variables. Based on more than 700 genetic variants that are associated with a higher risk of type 2 diabetes, Dr. Florez presented the key trait and variant associations for 5 clusters. The Beta-cell cluster (e.g., HNF1A, MTNR1B) is associated with lower insulin but higher proinsulin, likely due to defects in insulin processing and/or secretion. The Proinsulin cluster (e.g., ARAP1, SPRY2) is associated with lower levels of insulin and proinsulin, likely due to defects in insulin synthesis. The Obesity cluster (e.g., FTO, MC4R) is associated with higher levels of insulin and BMI, likely due to insulin resistance. The Lipodystrophy cluster (e.g., IRS1, PPARG) is associated with higher insulin but lower BMI, likely due to insulin resistance secondary to fat redistribution/ectopic fat accumulation. Lastly, Liver/Lipid cluster (e.g., GCKR, PNPLA3) is associated with higher insulin but lower triglycerides, likely due to defects in lipid metabolism. Dr. Florez further discussed how these genetic clusters are differentially distributed based on ethnic background, possibly explaining why certain populations, such as Asians, develop type 2 diabetes despite lower BMI. In the end, Dr. Florez concluded that genetics can serve as anchor subtypes, which need to be informed by physiology, and pathophysiological subtypes may help identify pathways for precision medicine.
-
![]()
August 6, 2025 (Session 4 - Part 1)
Session 3. “Why You Shouldn’t Drink Sweet Things” by Dr. Richard Lee (Harvard University)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
The DVSC25 continued today with 2 incredible Experts, Dr. Richard Lee from Harvard University and Dr. Jose Florez from Harvard Medical School. -
![]()
August 6, 2025 (Session 4 - Part 2)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
The DVC Team introduces Dr. Jose Florez to our Interns from all around the world. -
![]()
August 6, 2025 (Session 4 - Part 3)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 4)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Dr. Florez welcomes our program Interns from around the world. -
![]()
August 6, 2025 (Session 4 - Part 5)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Today’s exciting program continued with Dr. Jose Florez, who began by highlighting the heterogeneity of diabetes and an important finding from the Leif group showing how diabetic subjects could be clustered based on different clinical variables. Based on more than 700 genetic variants that are associated with a higher risk of type 2 diabetes, Dr. Florez presented the key trait and variant associations for 5 clusters. The Beta-cell cluster (e.g., HNF1A, MTNR1B) is associated with lower insulin but higher proinsulin, likely due to defects in insulin processing and/or secretion. The Proinsulin cluster (e.g., ARAP1, SPRY2) is associated with lower levels of insulin and proinsulin, likely due to defects in insulin synthesis. The Obesity cluster (e.g., FTO, MC4R) is associated with higher levels of insulin and BMI, likely due to insulin resistance. The Lipodystrophy cluster (e.g., IRS1, PPARG) is associated with higher insulin but lower BMI, likely due to insulin resistance secondary to fat redistribution/ectopic fat accumulation. Lastly, Liver/Lipid cluster (e.g., GCKR, PNPLA3) is associated with higher insulin but lower triglycerides, likely due to defects in lipid metabolism. Dr. Florez further discussed how these genetic clusters are differentially distributed based on ethnic background, possibly explaining why certain populations, such as Asians, develop type 2 diabetes despite lower BMI. In the end, Dr. Florez concluded that genetics can serve as anchor subtypes, which need to be informed by physiology, and pathophysiological subtypes may help identify pathways for precision medicine. -
![]()
August 6, 2025 (Session 4 - Part 6)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Jackson Professor of Clinical Medicine, Physician-in-Chief and Chair, Department of Medicine, Mass General Brigham Hospital, Harvard Medical School, and Institute Member, Broad Institute)
-
![]()
August 6, 2025 (Session 4 - Part 7)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Today’s exciting program continued with Dr. Jose Florez, who began by highlighting the heterogeneity of diabetes and an important finding from the Leif group showing how diabetic subjects could be clustered based on different clinical variables. -
![]()
August 6, 2025 (Session 4 - Part 8)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Today’s exciting program continued with Dr. Jose Florez, who began by highlighting the heterogeneity of diabetes and an important finding from the Leif group showing how diabetic subjects could be clustered based on different clinical variables. Based on more than 700 genetic variants that are associated with a higher risk of type 2 diabetes, Dr. Florez presented the key trait and variant associations for 5 clusters. The Beta-cell cluster (e.g., HNF1A, MTNR1B) is associated with lower insulin but higher proinsulin, likely due to defects in insulin processing and/or secretion. The Proinsulin cluster (e.g., ARAP1, SPRY2) is associated with lower levels of insulin and proinsulin, likely due to defects in insulin synthesis. The Obesity cluster (e.g., FTO, MC4R) is associated with higher levels of insulin and BMI, likely due to insulin resistance. The Lipodystrophy cluster (e.g., IRS1, PPARG) is associated with higher insulin but lower BMI, likely due to insulin resistance secondary to fat redistribution/ectopic fat accumulation. Lastly, Liver/Lipid cluster (e.g., GCKR, PNPLA3) is associated with higher insulin but lower triglycerides, likely due to defects in lipid metabolism. Dr. Florez further discussed how these genetic clusters are differentially distributed based on ethnic background, possibly explaining why certain populations, such as Asians, develop type 2 diabetes despite lower BMI. In the end, Dr. Florez concluded that genetics can serve as anchor subtypes, which need to be informed by physiology, and pathophysiological subtypes may help identify pathways for precision medicine. -
![]()
August 6, 2025 (Session 4 - Part 9)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 10)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 11)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Jackson Professor of Clinical Medicine, Physician-in-Chief and Chair, Department of Medicine, Mass General Brigham Hospital, Harvard Medical School, and Institute Member, Broad Institute)
-
![]()
August 6, 2025 (Session 4 - Part 12)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 13)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 14)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Today’s exciting program continued with Dr. Jose Florez, who began by highlighting the heterogeneity of diabetes and an important finding from the Leif group showing how diabetic subjects could be clustered based on different clinical variables. Based on more than 700 genetic variants that are associated with a higher risk of type 2 diabetes, Dr. Florez presented the key trait and variant associations for 5 clusters. The Beta-cell cluster (e.g., HNF1A, MTNR1B) is associated with lower insulin but higher proinsulin, likely due to defects in insulin processing and/or secretion. The Proinsulin cluster (e.g., ARAP1, SPRY2) is associated with lower levels of insulin and proinsulin, likely due to defects in insulin synthesis. The Obesity cluster (e.g., FTO, MC4R) is associated with higher levels of insulin and BMI, likely due to insulin resistance. The Lipodystrophy cluster (e.g., IRS1, PPARG) is associated with higher insulin but lower BMI, likely due to insulin resistance secondary to fat redistribution/ectopic fat accumulation. Lastly, Liver/Lipid cluster (e.g., GCKR, PNPLA3) is associated with higher insulin but lower triglycerides, likely due to defects in lipid metabolism. Dr. Florez further discussed how these genetic clusters are differentially distributed based on ethnic background, possibly explaining why certain populations, such as Asians, develop type 2 diabetes despite lower BMI. In the end, Dr. Florez concluded that genetics can serve as anchor subtypes, which need to be informed by physiology, and pathophysiological subtypes may help identify pathways for precision medicine. -
![]()
August 6, 2025 (Session 4 - Part 15)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 16)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 17)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Today’s exciting program continued with Dr. Jose Florez, who began by highlighting the heterogeneity of diabetes and an important finding from the Leif group showing how diabetic subjects could be clustered based on different clinical variables. Based on more than 700 genetic variants that are associated with a higher risk of type 2 diabetes, Dr. Florez presented the key trait and variant associations for 5 clusters. The Beta-cell cluster (e.g., HNF1A, MTNR1B) is associated with lower insulin but higher proinsulin, likely due to defects in insulin processing and/or secretion. The Proinsulin cluster (e.g., ARAP1, SPRY2) is associated with lower levels of insulin and proinsulin, likely due to defects in insulin synthesis. The Obesity cluster (e.g., FTO, MC4R) is associated with higher levels of insulin and BMI, likely due to insulin resistance. The Lipodystrophy cluster (e.g., IRS1, PPARG) is associated with higher insulin but lower BMI, likely due to insulin resistance secondary to fat redistribution/ectopic fat accumulation. Lastly, Liver/Lipid cluster (e.g., GCKR, PNPLA3) is associated with higher insulin but lower triglycerides, likely due to defects in lipid metabolism. Dr. Florez further discussed how these genetic clusters are differentially distributed based on ethnic background, possibly explaining why certain populations, such as Asians, develop type 2 diabetes despite lower BMI. In the end, Dr. Florez concluded that genetics can serve as anchor subtypes, which need to be informed by physiology, and pathophysiological subtypes may help identify pathways for precision medicine. -
![]()
August 6, 2025 (Session 4 - Part 18)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 19)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 20)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Today’s exciting program continued with Dr. Jose Florez, who began by highlighting the heterogeneity of diabetes and an important finding from the Leif group showing how diabetic subjects could be clustered based on different clinical variables. Based on more than 700 genetic variants that are associated with a higher risk of type 2 diabetes, Dr. Florez presented the key trait and variant associations for 5 clusters. The Beta-cell cluster (e.g., HNF1A, MTNR1B) is associated with lower insulin but higher proinsulin, likely due to defects in insulin processing and/or secretion. The Proinsulin cluster (e.g., ARAP1, SPRY2) is associated with lower levels of insulin and proinsulin, likely due to defects in insulin synthesis. The Obesity cluster (e.g., FTO, MC4R) is associated with higher levels of insulin and BMI, likely due to insulin resistance. The Lipodystrophy cluster (e.g., IRS1, PPARG) is associated with higher insulin but lower BMI, likely due to insulin resistance secondary to fat redistribution/ectopic fat accumulation. Lastly, Liver/Lipid cluster (e.g., GCKR, PNPLA3) is associated with higher insulin but lower triglycerides, likely due to defects in lipid metabolism. Dr. Florez further discussed how these genetic clusters are differentially distributed based on ethnic background, possibly explaining why certain populations, such as Asians, develop type 2 diabetes despite lower BMI. In the end, Dr. Florez concluded that genetics can serve as anchor subtypes, which need to be informed by physiology, and pathophysiological subtypes may help identify pathways for precision medicine. -
![]()
August 6, 2025 (Session 4 - Part 21)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 22)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Today’s exciting program continued with Dr. Jose Florez, who began by highlighting the heterogeneity of diabetes and an important finding from the Leif group showing how diabetic subjects could be clustered based on different clinical variables. Based on more than 700 genetic variants that are associated with a higher risk of type 2 diabetes, Dr. Florez presented the key trait and variant associations for 5 clusters. The Beta-cell cluster (e.g., HNF1A, MTNR1B) is associated with lower insulin but higher proinsulin, likely due to defects in insulin processing and/or secretion. The Proinsulin cluster (e.g., ARAP1, SPRY2) is associated with lower levels of insulin and proinsulin, likely due to defects in insulin synthesis. The Obesity cluster (e.g., FTO, MC4R) is associated with higher levels of insulin and BMI, likely due to insulin resistance. The Lipodystrophy cluster (e.g., IRS1, PPARG) is associated with higher insulin but lower BMI, likely due to insulin resistance secondary to fat redistribution/ectopic fat accumulation. Lastly, Liver/Lipid cluster (e.g., GCKR, PNPLA3) is associated with higher insulin but lower triglycerides, likely due to defects in lipid metabolism. Dr. Florez further discussed how these genetic clusters are differentially distributed based on ethnic background, possibly explaining why certain populations, such as Asians, develop type 2 diabetes despite lower BMI. In the end, Dr. Florez concluded that genetics can serve as anchor subtypes, which need to be informed by physiology, and pathophysiological subtypes may help identify pathways for precision medicine. -
![]()
August 6, 2025 (Session 4 - Part 23)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 24)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Type 2 diabetes” is a heterogeneous construct – a grab-basket of various disease processes designed to capture hyperglycemia that does not have a monogenic or autoimmune etiology. This presentation will review recent attempts to subcategorize type 2 diabetes using clinical, genomic or other data, aiming to deploy therapeutic interventions that target the underlying pathophysiology of the patient being treated. -
![]()
August 6, 2025 (Session 4 - Part 25)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Today’s exciting program continued with Dr. Jose Florez, who began by highlighting the heterogeneity of diabetes and an important finding from the Leif group showing how diabetic subjects could be clustered based on different clinical variables. Based on more than 700 genetic variants that are associated with a higher risk of type 2 diabetes, Dr. Florez presented the key trait and variant associations for 5 clusters. The Beta-cell cluster (e.g., HNF1A, MTNR1B) is associated with lower insulin but higher proinsulin, likely due to defects in insulin processing and/or secretion. The Proinsulin cluster (e.g., ARAP1, SPRY2) is associated with lower levels of insulin and proinsulin, likely due to defects in insulin synthesis. The Obesity cluster (e.g., FTO, MC4R) is associated with higher levels of insulin and BMI, likely due to insulin resistance. The Lipodystrophy cluster (e.g., IRS1, PPARG) is associated with higher insulin but lower BMI, likely due to insulin resistance secondary to fat redistribution/ectopic fat accumulation. Lastly, Liver/Lipid cluster (e.g., GCKR, PNPLA3) is associated with higher insulin but lower triglycerides, likely due to defects in lipid metabolism. Dr. Florez further discussed how these genetic clusters are differentially distributed based on ethnic background, possibly explaining why certain populations, such as Asians, develop type 2 diabetes despite lower BMI. In the end, Dr. Florez concluded that genetics can serve as anchor subtypes, which need to be informed by physiology, and pathophysiological subtypes may help identify pathways for precision medicine. -
![]()
August 6, 2025 (Session 4 - Part 26)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Lauren moderates the Q&A period as Dr. Florez addresses thoughtful questions from our interns. -
![]()
August 6, 2025 (Session 4 - Part 27)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
Dr. Florez addresses thoughtful questions from our interns. -
![]()
August 6, 2025 (Session 4 - Part 28)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
The DVC Team and our Interns thank Dr. Florez for a fantastic presentation. -
![]()
August 6, 2025 (Session 4 - Part 29)
Session 3-4. Open Mic Session by the DVC Team.
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 6, 2025 (Session 4 - Part 30)
Session 3-4. Open Mic Session by the DVC Team.
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 6, 2025 (Session 4 - Part 31)
Session 3-4. Open Mic Session by the DVC Team.
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 6, 2025 (Session 4 - Part 32)
Session 4. “Precision Medicine in Diabetes” by Dr. Jose Florez (Harvard Medical School)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 8, 2025
Session 5. Role of Social Determinants of Health in Diabetes Care by Dr. Enrique Caballero (Harvard Medical School)
The DVSC25 continued today with 2 inspirational Experts, Dr. Enrique Caballero from Harvard Medical School and Dr. Maureen Gannon from the Vanderbilt University School of Medicine. Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices.
-
![]()
August 8, 2025 (Session 5 - Part 1)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)The DVSC25 continued today with 2 inspirational Experts, Dr. Enrique Caballero from Harvard Medical School and Dr. Maureen Gannon from the Vanderbilt University School of Medicine, joined by Caroline Blanco (Senior Director of Professional Engagement) from the American Diabetes Association.
-
![]()
August 8, 2025 (Session 5 - Part 2)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
The DVC Team introduces Dr. Enrique Caballero to our Interns from all around the world. -
![]()
August 8, 2025 (Session 5 - Part 3)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
This session will allow participants to identify the role of social determinants of health in the development and course of diabetes. Important aspects such as food insecurity, lack of health care access, limited access to medications, scarce social support, and some environmental issues will be discussed. -
![]()
August 8, 2025 (Session 5 - Part 4)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero welcomes our program interns from around the world. -
![]()
August 8, 2025 (Session 5 - Part 5)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero welcomes our program interns from around the world. -
![]()
August 8, 2025 (Session 5 - Part 6)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Associate Professor of Medicine, Director, International Innovation Program and Latino Diabetes Health, Brigham & Women’s Hospital, Harvard Medical School, and President-Elect, Medicine & Science, American Diabetes Association)
-
![]()
August 8, 2025 (Session 5 - Part 7)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. -
![]()
August 8, 2025 (Session 5 - Part 8)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 9)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 10)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 11)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 12)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 13)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 14)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 15)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 16)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 17)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 18)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 19)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 20)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 21)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 22)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 23)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 24)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 25)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 26)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 27)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 28)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 29)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 30)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 31)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 32)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero began with the basic triad in diabetes care, involving the healthcare system, healthcare provider, and patient. An important variable in diabetes care lies in the social determinants of health: socioeconomic status (education, income, occupation), neighborhood and physical environment (housing, built environment, toxic environmental exposures), food environment (food insecurity, food access, food availability, food affordability), health care (access, affordability, quality), and social context (social cohesion, social capital, social support). Dr. Caballero highlighted how the same area with high diabetes prevalence is the same area with high food deserts, indicating the importance of assessing food insecurity by healthcare providers. Dr. Caballero shared how he discusses with his patients about nutrition and food resources and guides their access to healthcare, offering comprehensive care necessary to tackle a multifactorial disease, such as diabetes. He also shared some creative approaches involving group medical visits where patients learn from each other and peer support intervention, involving the community. In all, Dr. Caballero summarized the multiple voices in diabetes care, extending beyond the basic triad and including government policies, social structure, educational institutions, professional organizations, patient advocacy groups, non-profit and community-based organizations, health insurance companies, and manufacturers of pharmaceuticals and devices. -
![]()
August 8, 2025 (Session 5 - Part 33)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Lauren moderates the Q&A period as Dr. Caballero addresses thoughtful questions from our interns. -
![]()
August 8, 2025 (Session 5 - Part 34)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero addresses thoughtful questions from our interns. -
![]()
August 8, 2025 (Session 5 - Part 35)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero addresses thoughtful questions from our interns. -
![]()
August 8, 2025 (Session 5 - Part 36)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero addresses thoughtful questions from our interns. -
![]()
August 8, 2025 (Session 5 - Part 37)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Lauren moderates the Q&A period as Dr. Caballero addresses thoughtful questions from our interns. -
![]()
August 8, 2025 (Session 5 - Part 38)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
Dr. Caballero addresses thoughtful questions from our interns. -
![]()
August 8, 2025 (Session 5 - Part 39)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
The DVC Team and our Interns thank Dr. Caballero for a fantastic presentation. -
![]()
August 8, 2025 (Session 5 - Part 40)
Session 5. “Role of Social Determinants of Health in Diabetes Care” by Dr. Enrique Caballero (Harvard Medical School)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 8, 2025
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Caroline Blanco, Senior Director of Professional Engagement, introducing her role for the American Diabetes Association and celebrating our global audience. This was followed by Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes.
-
![]()
August 8, 2025 (Session 6 - Part 1)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Caroline Blanco, Senior Director of Professional Engagement, introducing her role for the American Diabetes Association and celebrating our global audience. -
![]()
August 8, 2025 (Session 6 - Part 2)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
The DVC Team introduces Dr. Maureen Gannon to our Interns from all around the world. -
![]()
August 8, 2025 (Session 6 - Part 3)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
The DVC Team introduces Dr. Maureen Gannon to our Interns from all around the world. -
![]()
August 8, 2025 (Session 6 - Part 4)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
The goal of my presentation is to discuss how your life’s experiences shape who you are. I will discuss the importance of having great mentors and making the most of opportunities and curveballs that come your way. I will also talk about following where the science takes you and not being afraid to learn new things. I will end by describing the main biological questions and methods that my lab addresses and utilizes in our attempts to find therapies to prevent and treat diabetes. -
![]()
August 8, 2025 (Session 6 - Part 5)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Dr. Maureen Gannon welcomes our Interns from all around the world. -
![]()
August 8, 2025 (Session 6 - Part 6)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Dr. Maureen Gannon welcomes our Interns from all around the world. -
![]()
August 8, 2025 (Session 6 - Part 7)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Professor of Medicine, Professor of Molecular Physiology & Biophysics and Cell & Developmental Biology, and Associate Dean for Faculty Development, Vanderbilt University School of Medicine)
-
![]()
August 8, 2025 (Session 6 - Part 8)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 9)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 10)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 11)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 12)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 13)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 14)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 15)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 16)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 17)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 18)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 19)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 20)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 21)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 22)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 23)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 24)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 25)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 26)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Today’s exciting program continued with Dr. Maureen Gannon, our 2021 Expert, whose inspirational presentation still reverberates in the minds of many of our past interns, graciously returning to lead today’s session. Dr. Gannon began by sharing how “Everyone has had different life experiences and different paths. The path you walk shapes how you see the world and what you bring to the scientific process. She shared her humble beginning and the many twists and turns throughout her journey, some unexpected, yet she made the most out of her opportunities, leading her to success. What an inspiring story and priceless advice for our interns! She then discussed her lifelong research on studying different ways to increase the functional beta-cell mass, discovering several pathways that can increase beta-cell mass and stimulate beta-cell proliferation, highlighting the role of the Foxm1 transcription factor, which decreases with age. Her research found that FoxM1 activation enhanced beta-cell proliferation in aging mice, and FoxM1 also protected beta-cells against cytokines. She further showed how prostaglandin E2 receptors, EP3 and EP4, had opposing effects on beta-cell survival and proliferation. Dr. Gannon summarized how combined EP3 antagonism EP4 agonism preserves beta-cell mass and may prevent or delay type 1 diabetes. -
![]()
August 8, 2025 (Session 6 - Part 27)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Lauren moderates the Q&A period as Dr. Gannon addresses thoughtful questions from our interns. -
![]()
August 8, 2025 (Session 6 - Part 28)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Dr. Gannon addresses thoughtful questions from our interns. -
![]()
August 8, 2025 (Session 6 - Part 29)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Lauren moderates the Q&A period as Dr. Gannon addresses thoughtful questions from our interns. -
![]()
August 8, 2025 (Session 6 - Part 30)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
Dr. Gannon addresses thoughtful questions from our interns. -
![]()
August 8, 2025 (Session 6 - Part 31)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
The DVC Team and our Interns thank Dr. Gannon for a fantastic presentation. -
![]()
August 8, 2025 (Session 6 - Part 32)
Session 5-6: Pre-Session Q&A by the DVC Team.
Dr. Kim invites our interns to ask questions during Pre-Session Q&A period. -
![]()
August 8, 2025 (Session 6 - Part 33)
Session 5-6: Open Mic Session by the DVC Team.
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 8, 2025 (Session 6 - Part 34)
Session 5-6: Open Mic Session by the DVC Team.
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 8, 2025 (Session 6 - Part 35)
Session 6. “How Did I Get Here? One Scientist’s Journey” by Dr. Maureen Gannon (Vanderbilt University School of Medicine)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 11, 2025
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
The DVSC25 continued today with more informative and exciting sessions from Dr. Jason Kim from the University of Massachusetts Chan Medical School and Dr. Vasilis Vasiliou from the Yale School of Public Health.
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research.
-
![]()
August 11, 2025 (Session 7 - Part 1)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)The DVSC25 continued today with more informative and exciting sessions from Dr. Jason Kim from the University of Massachusetts Chan Medical School and Dr. Vasilis Vasiliou from the Yale School of Public Health.
-
![]()
August 11, 2025 (Session 7 - Part 2)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim welcomes our program interns from around the world. -
![]()
August 11, 2025 (Session 7 - Part 3)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
This session introduces how high school and college students can explore their interests in science and medicine, and how college activities can be organized to prepare for medical school and graduate school. The session presents the admissions process for medical school and graduate school and their curriculum, clinical residency and fellowship programs, and academic tenure process. The session further discusses how to develop a research idea into a fundable project, write an NIH grant, and succeed at different stages of an academic career. The session ends with challenges that lie ahead in academic medicine and research, and the passion and service that define this lifelong profession. This session supports people at all stages of their educational and professional careers. -
![]()
August 11, 2025 (Session 7 - Part 4)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 5)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (Professor of Molecular Medicine, Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, and MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
-
![]()
August 11, 2025 (Session 7 - Part 6)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 7)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 8)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 9)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 10)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 11)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 12)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 13)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 14)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 15)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 16)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 17)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 18)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 19)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 20)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 21)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by illustrating how medical education is not for everyone due to its high cost, and should be pursued by one’s passion and strong interest. He noted how graduation from a medical school offers many different career paths. For high school and college students aspiring to be physicians, he discussed what they can do to prepare for medical school. He discussed the medical school application process and gave insightful advice on college activities/experiences and interviews. He also described the differences between the MD and MD/PhD dual programs. For medical students and residents, Dr. Kim shared a curriculum for the gastroenterology and hepatology fellowship training program at the University of Massachusetts Chan Medical School. He then discussed the importance of research in clinical training and elaborated on the investigative approach to science and medicine, including helpful tips on how to critically assess peer-reviewed research articles when searching for scientific information. For clinical fellows and early-career scientists and clinicians, Dr. Kim discussed what institutions look for in hiring new faculty, the tenure process, and professorship in academic medicine. He gave an example of what a day in the life of a professor would look like with respect to the administrative, teaching, clinical, and research roles for the institution and outside the institution at the national and international levels. Dr. Kim discussed what an early-career investigator should do to establish a productive research program and further shared insightful tips on writing an NIH grant (Grant Writing 101!). Dr. Kim ended his presentation by highlighting the University of Massachusetts Chan Medical School as a leading medical institution with outstanding patient care, education, and research. -
![]()
August 11, 2025 (Session 7 - Part 22)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim invites our interns to ask questions. -
![]()
August 11, 2025 (Session 7 - Part 23)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Lauren moderates the Q&A period as Dr. Kim addresses thoughtful questions from our interns. -
![]()
August 11, 2025 (Session 7 - Part 24)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim addresses thoughtful questions from our interns. -
![]()
August 11, 2025 (Session 7 - Part 25)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
The DVC Team and our Interns thank Dr. Kim for a fantastic presentation. -
![]()
August 11, 2025 (Session 7 - Part 26)
Session 7. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 11, 2025
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas.
-
![]()
August 11, 2025 (Session 8 - Part 1)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
The DVC Team introduces Dr. Vasiliou to our Interns from all around the world. -
![]()
August 11, 2025 (Session 8 - Part 2)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou welcomes our program interns from around the world. -
![]()
August 11, 2025 (Session 8 - Part 3)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
This presentation will explore how variations in glutathione (GSH) levels influence the development and progression of diabetes. Drawing from experimental models, the talk highlights the paradoxical roles of GSH—where low levels may offer protection while complete deficiency leads to disease. We will discuss the interplay between redox balance, oxidative stress, and metabolic regulation, offering new insights into redox-targeted interventions for diabetes. -
![]()
August 11, 2025 (Session 8 - Part 4)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 5)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 6)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Department Chair and Susan Dwight Bliss Professor of Epidemiology, Yale School of Public Health, Professor of Ophthalmology & Visual Sciences, Yale University School of Medicine, Professor, Yale School of Environment, and Director, Yale Superfund Research Center)
-
![]()
August 11, 2025 (Session 8 - Part 7)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 8)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 9)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 10)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 11)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 12)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 13)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 14)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 15)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 16)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 17)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 18)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 19)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 20)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 21)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 22)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 23)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 24)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 25)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 26)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 27)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 28)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 29)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 30)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 31)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou began with an introduction to the glutathione (GSH) spectrum as a double-edged sword in redox biology, describing GSH in diabetes and redox biology in metabolism. He discussed the important role of oxidative stress in human diseases, highlighting low GSH levels in the pathogenesis of fatty liver disease, and the protective role of the antioxidant system. Catalase is an antioxidant enzyme, and his earlier research found that the loss of catalase can promote prediabetic and obesity phenotypes. Dr. Vasiliou showed how a conditional deletion of glutamate-cysteine ligase catalytic (Gclc) subunit, the rate-limiting step in GSH biosynthesis, in the pancreas results in a severe diabetes phenotype at an early age due to loss of insulin-producing beta-cells and defects in glucose-stimulated insulin secretion. These effects were associated with oxidative stress in their islets. Using a tamoxifen-inducible approach to ablate the Gclc gene in later life, Dr. Vasiliou found that mice initially developed hyperinsulinemia that was followed by hyperglycemia and delayed diabetes phenotypes. The early preservation of redox balance despite reduced GSH levels may be due to compensatory mechanisms that are insufficient to maintain beta-cell function over time. In all, these exciting findings from Dr. Vailiou’s laboratory offer new insight into the role of GSH biosynthesis in maintaining beta-cell health in the adult pancreas. -
![]()
August 11, 2025 (Session 8 - Part 32)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Lauren moderates the Q&A period as Dr. Vasiliou addresses thoughtful questions from our interns. -
![]()
August 11, 2025 (Session 8 - Part 33)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou addresses thoughtful questions from our interns. -
![]()
August 11, 2025 (Session 8 - Part 34)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Lauren moderates the Q&A period as Dr. Vasiliou addresses thoughtful questions from our interns. -
![]()
August 11, 2025 (Session 8 - Part 35)
Session 8. “Redox Biology in Diabetes: Lessons from the Glutathione Spectrum” by Dr. Vasilis Vasiliou (Yale School of Public Health)
Dr. Vasiliou addresses thoughtful questions from our interns. -
![]()
August 11, 2025 (Session 8 - Part 36)
Session 7-8: Open Mic Session by Dr. Jason Kim and DVC Team.
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 11, 2025 (Session 8 - Part 36)
Session 7-8: Open Mic Session by Dr. Jason Kim and DVC Team.
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 11, 2025 (Session 8 - Part 37)
Session 7-8: Open Mic Session by Dr. Jason Kim and DVC Team.
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 11, 2025 (Session 8 - Part 38)
Session 8. “Preparing for a Career in Science and Medicine” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 13, 2025
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
The DVSC25 continued today with more inspiring and exciting sessions from Dr. Bob Gabbay from Harvard Medical School and Dr. Jean Schaffer from Harvard Medical School.
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed!
-
![]()
August 13, 2025 (Session 9 - Part 1)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)The DVSC25 continued today with more inspiring and exciting sessions from Dr. Bob Gabbay from Harvard Medical School and Dr. Jean Schaffer from Harvard Medical School.
-
![]()
August 13, 2025 (Session 9 - Part 2)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)The DVSC25 continued today with more inspiring and exciting sessions from Dr. Bob Gabbay from Harvard Medical School and Dr. Jean Schaffer from Harvard Medical School.
-
![]()
August 13, 2025 (Session 9 - Part 3)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
The DVC Team introduces Dr. Gabbay to our Interns from all around the world. -
![]()
August 13, 2025 (Session 9 - Part 4)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
The DVC Team introduces Dr. Gabbay to our Interns from all around the world. -
![]()
August 13, 2025 (Session 9 - Part 5)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
The field of diabetes research and care is vast. As I reflect on the nearly 40-year journey, I will share the drive to improve the lives of people affected by diabetes and maximize impact. It has been a journey from the micro to the macro. Along the way, you can see the many opportunities that being involved in the world of diabetes presents for your future career journeys. -
![]()
August 13, 2025 (Session 9 - Part 6)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay welcomes our program interns from around the world. -
![]()
August 13, 2025 (Session 9 - Part 7)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Associate Professor of Medicine, Former Chief Medical Officer, Joslin Diabetes Center, Harvard Medical School, and Former Chief Scientific and Medical Officer, American Diabetes Association)
-
![]()
August 13, 2025 (Session 9 - Part 8)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 9)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 10)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 11)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 12)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 13)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 14)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 15)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 16)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 17)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 18)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 19)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 20)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 21)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 22)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 23)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 24)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 25)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 26)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 27)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 28)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 29)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 30)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 31)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 32)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 33)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 34)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 35)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 36)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay began by asking our interns to describe what diabetes means to them in one word. Some of the answers from our interns were very insightful: “everywhere”, “long-term”, “epidemic”, “preventable”, “complex”, “life-changing”, “treatable”, “stressful”, “heterogeneous”, and “multifaceted.” Dr. Gabbay shared the story of his own journey, beginning with graduate education and research that led to medical school and clinical training with a growing desire to interact with patients. He then realized how he wanted to do more than just care for a patient and wanted to make an impact on population health, which became possible upon joining the Joslin Diabetes Center as Chief Medical Officer, overseeing more than 25,000 patients. He was able to make an even greater impact upon joining the American Diabetes Association as Chief Scientific and Medical Officer, leading the ADA’s global effort to drive discovery within the world of diabetes research, care, and prevention. In addition to the roles played by a researcher, clinician, and educator, Dr. Gabbay discussed how one can have an impact on diabetes through digital health, data science, drug discovery, device development, leadership, health writing, content creation, entrepreneurship, government, and policy. He ended with some helpful tips for our interns: reach out to people who are doing what you want to do someday, and find out the pros and cons of their work and what their day in the life is like. He also advised our interns to say “yes” to opportunities because one never knows where an opportunity may lead. A very perceptive advice indeed! -
![]()
August 13, 2025 (Session 9 - Part 37)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay invites our interns to ask questions. -
![]()
August 13, 2025 (Session 9 - Part 38)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Lauren moderates the Q&A period as Dr. Gabbay addresses thoughtful questions from our interns. -
![]()
August 13, 2025 (Session 9 - Part 39)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay addresses thoughtful questions from our interns. -
![]()
August 13, 2025 (Session 9 - Part 40)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay addresses thoughtful questions from our interns. -
![]()
August 13, 2025 (Session 9 - Part 41)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay invites our interns to ask questions. -
![]()
August 13, 2025 (Session 9 - Part 42)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Lauren moderates the Q&A period as Dr. Gabbay addresses thoughtful questions from our interns. -
![]()
August 13, 2025 (Session 9 - Part 43)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay addresses thoughtful questions from our interns. -
![]()
August 13, 2025 (Session 9 - Part 44)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay addresses thoughtful questions from our interns. -
![]()
August 13, 2025 (Session 9 - Part 45)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Dr. Gabbay addresses thoughtful questions from our interns. -
![]()
August 13, 2025 (Session 9 - Part 46)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
The DVC Team and our Interns thank Dr. Gabbay for a fantastic presentation. -
![]()
August 13, 2025 (Session 9 - Part 47)
Session 9-10: Pre-Session Q&A by Dr. Jason Kim and the DVC Team.
Dr. Kim invites our interns to ask questions during the Pre-Session Q&A period. -
![]()
August 13, 2025 (Session 9 - Part 48)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 13, 2025
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity.
-
![]()
August 13, 2025 (Session 10 - Part 1)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)The DVSC25 continued today with more inspiring and exciting sessions from Dr. Bob Gabbay from Harvard Medical School and Dr. Jean Schaffer from Harvard Medical School.
-
![]()
August 13, 2025 (Session 10 - Part 2)
Session 9. “A Quest for Impact” by Dr. Robert Gabbay (Harvard Medical School)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)The DVSC25 continued today with more inspiring and exciting sessions from Dr. Bob Gabbay from Harvard Medical School and Dr. Jean Schaffer from Harvard Medical School.
-
![]()
August 13, 2025 (Session 10 - Part 3)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
The DVC Team introduces Dr. Schaffer to our Interns from all around the world. -
![]()
August 13, 2025 (Session 10 - Part 4)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
The DVC Team introduces Dr. Schaffer to our Interns from all around the world. -
![]()
August 13, 2025 (Session 10 - Part 5)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
The DVC Team introduces Dr. Schaffer to our Interns from all around the world. -
![]()
August 13, 2025 (Session 10 - Part 6)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
High blood sugar is a cardinal feature of diabetes. This talk will focus on how high levels of blood glucose alters cellular functions and contributes to the pathogenesis of diabetes. -
![]()
August 13, 2025 (Session 10 - Part 7)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 8)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Professor of Medicine, Senior Investigator, and Associate Research Director, Joslin Diabetes Center, Harvard Medical School)
-
![]()
August 13, 2025 (Session 10 - Part 9)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 10)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 11)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 12)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 13)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 14)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 15)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 16)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 17)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 18)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 19)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 20)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 21)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 22)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 23)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 24)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 25)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 26)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 27)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 28)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 29)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 30)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 31)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 32)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 33)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 34)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 35)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 36)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 37)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 38)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer began by outlining the role of hyperglycemia in type 1 and type 2 diabetes and the biochemical and genetic consequences of hyperglycemia. She highlighted how, despite recent advances in diabetes treatment, hyperglycemia remains common, as half of American adults with diabetes fail to meet HbA1C targets. This is critical since intensive insulin therapy and better glucose monitoring improve beta-cell function and reduce diabetic complications in people with type 1 and type 2 diabetes. Dr. Schaffer introduced how excess glucose can be metabolized through the aldose reductase pathway and the hexosamine pathway, leading to oxidative stress in the cells. Hyperglycemia can also increase advanced glycation end-products and lipid intermediates that are known to activate deleterious signaling cascades involved in diabetic complications. Dr. Schaffer discussed how hyperglycemia can regulate mRNA production by impacting chromatin confirmation and activity of transcriptional factors, one example being glucose regulation of ChREBP. She further discussed how not only does glucose stimulate insulin secretion by the beta-cells, glucose also increases insulin synthesis. Importantly, Dr. Schaffer’s laboratory found that chronic glucose inhibits glucose-stimulated insulin secretion by affecting many genes critical for glucose metabolism and insulin processing in the beta-cells at the level of mRNA translation. In all, Dr. Schaffer is elucidating the mechanism by which translational machinery, in particular ribosomes, may be affected by nutrients and developing novel therapeutics to interrupt glucotoxicity. -
![]()
August 13, 2025 (Session 10 - Part 39)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Lauren moderates the Q&A period as Dr. Schaffer addresses thoughtful questions from our interns. -
![]()
August 13, 2025 (Session 10 - Part 40)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer addresses thoughtful questions from our interns. -
![]()
August 13, 2025 (Session 10 - Part 41)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
Dr. Schaffer addresses thoughtful questions from our interns. -
![]()
August 13, 2025 (Session 10 - Part 42)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
The DVC Team and our Interns thank Dr. Schaffer for a fantastic presentation. -
![]()
August 13, 2025 (Session 10 - Part 43)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
The DVC Team and our Interns thank Dr. Schaffer for a fantastic presentation. -
![]()
August 13, 2025 (Session 10 - Part 44)
Session 9-10: Open Mic Session by the DVC Team
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 13, 2025 (Session 10 - Part 45)
Session 9-10: Open Mic Session by the DVC Team
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 13, 2025 (Session 10 - Part 46)
Session 9-10: Open Mic Session by the DVC Team
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 13, 2025 (Session 10 - Part 47)
Session 9-10: Open Mic Session by the DVC Team
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 13, 2025 (Session 10 - Part 48)
Session 9-10: Open Mic Session by the DVC Team
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 13, 2025 (Session 10 - Part 49)
Session 9-10: Open Mic Session by the DVC Team
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 13, 2025 (Session 10 - Part 50)
Session 9-10: Open Mic Session by the DVC Team
The DVC team invites our interns to ask questions and engage in a discussion at the interactive Open Mic Forum. -
![]()
August 13, 2025 (Session 10 - Part 51)
Session 10. “Glucose Excess in Diabetes Pathogenesis” by Dr. Jean Schaffer (Harvard Medical School)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 15, 2025
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
After 2 weeks of exciting and inspiring sessions from the Experts, we have reached the finale of this year’s program. Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes!
-
![]()
August 15, 2025 (Session 11 - Part 1)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)After 2 weeks of exciting and inspiring sessions, we have reached the finale of this year’s program.
-
![]()
August 15, 2025 (Session 11 - Part 2)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
The DVC Team introduces Dr. Mauvais-Jarvis to our Interns from all around the world. -
![]()
August 15, 2025 (Session 11 - Part 3)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
The DVC Team introduces Dr. Mauvais-Jarvis to our Interns from all around the world. -
![]()
August 15, 2025 (Session 11 - Part 4)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis welcomes our program interns from around the world. -
![]()
August 15, 2025 (Session 11 - Part 5)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
I will discuss evidence from clinical studies and basic science investigations that estradiol (E2) and testosterone (T) are produced in males and females and are potent metabolic regulators in both sexes. When E2 and T production decreases during aging, metabolic dysfunction develops and predisposes to metabolic syndrome and type 2 diabetes. Optimizing levels of both hormones protects men and women from cardiometabolic disease. -
![]()
August 15, 2025 (Session 11 - Part 6)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Price-Goldsmith Professor of Nutrition, Professor of Medicine, Section of Endocrinology & Metabolism, Director, Center of Excellence in Sex-Based Precision Medicine, and Director, Hormones & Metabolism Discovery Research Laboratory, Tulane University School of Medicine)
-
![]()
August 15, 2025 (Session 11 - Part 7)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 8)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 9)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 10)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 11)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 12)
Session 2: Introduction to Diabetes by Dr. Jason Kim (Professor of Molecular Medicine, Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
-
![]()
August 15, 2025 (Session 11 - Part 13)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 14)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 15)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 16)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 17)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 18)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 19)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 20)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 21)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 22)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 23)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 24)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 25)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 26)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 27)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 28)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 29)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 30)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 31)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 32)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 33)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 34)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 35)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 36)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 37)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 38)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 39)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 40)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 41)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 42)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 43)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 44)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 45)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 46)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 47)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 48)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 49)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 50)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 51)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis began by illustrating how sex is a genetic modifier of human diseases. Estradiol and testosterone are key metabolic hormones in females, with estrogens playing a potent role in regulating insulin sensitivity and glucose homeostasis. Interestingly, all autoimmune disorders have a female bias in prevalence, except for type 1 diabetes, which may relate to estrogen’s protective effects on beta-cell function and survival in females. Estradiol was also shown to improve human islet engraftment and revascularization in insulin-deficient diabetic mice. Dr. Mauvais-Jarvis further discussed how estrogens promote degradation of misfolded proinsulin, thereby protecting insulin production and delaying diabetes. Targeting estrogen delivery selectively to the brain and beta-cells was achieved using GLP-1-E2 fusion peptides, conferring metabolic protection via GLP-1R and ERa. Dr. Mauvais-Jarvis also discussed polycystic ovary syndrome, a topic of high interest to our interns. He then discussed how testosterone is an anti-diabetic hormone in men, and the testicular-islet axis uses testosterone to enhance GLP-1 action. He further presented how a conditional loss of androgen receptor in beta-cells resulted in type 2 diabetes phenotypes in mice, implicating an important role of testosterone in beta-cells. Dihydrotestosterone was shown to increase GLP-1 action as the androgen receptor interacts with GLP-1 receptor, resulting in increased GLP-1-mediated insulin secretion by the beta-cells. Dr. Mauvais-Jarvis ended by discussing how sex differences in human islet cell transcriptomes predominantly affect sex chromosome genes. Very interesting science on our sex hormones as they relate to diabetes! -
![]()
August 15, 2025 (Session 11 - Part 52)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis invites our interns to ask questions. -
![]()
August 15, 2025 (Session 11 - Part 53)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Lauren moderates the Q&A period as Dr. Mauvais-Jarvis addresses thoughtful questions from our interns. -
![]()
August 15, 2025 (Session 11 - Part 54)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis addresses thoughtful questions from our interns. -
![]()
August 15, 2025 (Session 11 - Part 55)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Allison moderates the Q&A period as Dr. Mauvais-Jarvis addresses thoughtful questions from our interns. -
![]()
August 15, 2025 (Session 11 - Part 56)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Dr. Mauvais-Jarvis addresses thoughtful questions from our interns. -
![]()
August 15, 2025 (Session 11 - Part 57)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
The DVC Team and our Interns thank Dr. Mauvais-Jarvis for a fantastic presentation. -
![]()
August 15, 2025 (Session 11 - Part 58)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
The DVC Team and our Interns thank Dr. Mauvais-Jarvis for a fantastic presentation. -
![]()
August 15, 2025 (Session 11 - Part 59)
Session 11-13: Pre-Session Q&A by the DVC Team.
Dr. Kim invites our interns to ask questions during the Pre-Session Q&A period. -
![]()
August 15, 2025 (Session 11 - Part 60)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 15, 2025
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
The Closing Sessions continued with a more exciting session on type 2 diabetes and one of its major comorbidities, fatty liver disease, from Dr. Jason Kim. Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes.
-
![]()
August 15, 2025 (Session 12 - Part 1)
Session 11. “Anti-Diabetic Effects of Estrogen and Testosterone in Women and Men” by Dr. Franck Mauvais-Jarvis (Tulane University School of Medicine)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)The Closing Sessions continued with a more exciting session on type 2 diabetes and one of its major comorbidities, fatty liver disease, from Dr. Jason Kim.
-
![]()
August 15, 2025 (Session 12 - Part 2)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim welcomes our program interns from around the world. -
![]()
August 15, 2025 (Session 12 - Part 3)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim welcomes our program interns from around the world. -
![]()
August 15, 2025 (Session 12 - Part 4)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
This session introduces the topic of obesity, multifactorial causes for the rising obesity rates, and the danger of excess weight gain during pregnancy. The session presents inflammation in obesity and the molecular link between inflammation, insulin resistance, and type 2 diabetes. The session further discusses fatty liver disease as a major comorbidity of diabetes and how inflammation is also involved in the progression of fatty liver to metabolic dysfunction-associated steatohepatitis (MASH). Our recent work published in Nature Communications will be highlighted. The session ends with our latest NIH-funded research studying an important connection between Alzheimer’s disease and diabetes. -
![]()
August 15, 2025 (Session 12 - Part 5)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (Professor of Molecular Medicine, Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, and MD/PhD Admissions Committee, University of Massachusetts Chan Medical School)
-
![]()
August 15, 2025 (Session 12 - Part 6)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 7)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 8)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 9)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 10)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 11)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 12)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 13)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 14)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 15)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 16)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 17)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 18)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim began by introducing key discoveries made in early 2000 with IL-6 regulating insulin action and macrophages infiltrating adipose tissue in obesity, shaping an exciting new paradigm on the role of inflammation in type 2 diabetes. He discussed how IL-10 is a major anti-inflammatory cytokine in our body, and muscle-selective overexpression of IL-10 rescued mice from insulin resistance associated with obesity and aging. This was due to IL-10’s effects to suppress obesity and aging-mediated inflammation in skeletal muscle. Dr. Kim further highlighted how excess weight gain is a common issue in pregnancy, and offspring mice from high-fat diet-fed females developed insulin resistance with inflammation in the liver, supporting the important notion that excess weight gain during pregnancy may contribute to gestational diabetes and intergenerational risk for diabetes. He then discussed metabolic dysfunction-associated steatotic liver disease and how IFNg signaling in macrophages regulates obesity-mediated inflammation and insulin resistance in the liver. Mice with a conditional loss of IFNg signaling in macrophages were protected from diet-induced insulin resistance in the liver and fatty liver progression to inflammation and fibrosis, implicating macrophage IFNg signaling as a critical checkpoint in the development of metabolic dysfunction-associated steatohepatitis. Dr. Kim ended by briefly sharing some preliminary data from recent projects focusing on the molecular relationship between Alzheimer’s disease and type 2 diabetes. -
![]()
August 15, 2025 (Session 12 - Part 19)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim invites our interns to ask questions. -
![]()
August 15, 2025 (Session 12 - Part 20)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Lauren moderates the Q&A period as Dr. Kim addresses thoughtful questions from our interns. -
![]()
August 15, 2025 (Session 12 - Part 21)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim addresses thoughtful questions from our interns. -
![]()
August 15, 2025 (Session 12 - Part 22)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Allison moderates the Q&A period as Dr. Kim addresses thoughtful questions from our interns. -
![]()
August 15, 2025 (Session 12 - Part 23)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
Dr. Kim addresses thoughtful questions from our interns. -
![]()
August 15, 2025 (Session 12 - Part 24)
Session 12. “Role of Inflammation in Type 2 Diabetes and Fatty Liver Disease” by Dr. Jason Kim (University of Massachusetts Chan Medical School)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.
August 15, 2025
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
After 13 exciting sessions of the Diabetes Virtual Summer Camp 2025, we arrived at the Closing Session with Lauren Kim (Founder, Program Director, and Webmaster), Allison Kim (Associate Director and Director of Outreach), and Dr. Jason Kim (Advisor). Lauren and Allison expressed heartfelt appreciation to the DVSC25 interns for their growing passion and commitment to diabetes research, education, and care. Our interns asked outstanding questions using the Chat box following each session, demonstrating vital traits of an investigator, observation, and inquiry. Their dedication to learning was demonstrated through the pre-session Q&A period and echoed during the open-mic forum. Dr. Kim explained the process of requesting a program certificate for the Diabetes Virtual Summer Camp 2025. The Diabetes Virtual Camp Team then administered electronic polls and surveys to collect important feedback from the interns about the program and explained the final program task to be completed within a week. Our interns didn’t want to leave as we held a final open-mic forum where the interns were unmuted to directly ask questions and provide more feedback on our program. The Closing Sessions ended with the final farewells to everyone as we remain hopeful that our program has met the goals to supporting the next generation of physicians and scientists and one day finding a cure for diabetes. The Diabetes Virtual Camp looks forward to seeing you all in 2026!
-
![]()
August 15, 2025 (Session 13 - Part 1)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
The DVC Team congratulates our interns for completing the Diabetes Virtual Summer Camp 2025! -
![]()
August 15, 2025 (Session 13 - Part 2)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’)
Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’)
Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School) -
![]()
August 15, 2025 (Session 13 - Part 3)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Lauren Kim thanks the audience for participating in the Diabetes Virtual Summer Camp 2025 and hopes that our program will be helpful to everyone’s journeys, while our new Networking page will continue to unite our passion for diabetes. -
![]()
August 15, 2025 (Session 13 - Part 4)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Allison Kim thanks the audience for participating in the Diabetes Virtual Summer Camp 2025 and hopes that our program will be helpful to everyone’s journeys, while our new Networking page will continue to unite our passion for diabetes. -
![]()
August 15, 2025 (Session 13 - Part 5)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Dr. Jason Kim thanks the audience for participating in the Diabetes Virtual Summer Camp 2025 and hopes that our program will be helpful to everyone’s journeys, while our new Networking page will continue to unite our passion for diabetes. -
![]()
August 15, 2025 (Session 13 - Part 6)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
After 13 exciting sessions of the Diabetes Virtual Summer Camp 2025, we arrived at the Closing Session with Lauren Kim (Founder, Program Director, and Webmaster), Allison Kim (Associate Director and Director of Outreach), and Dr. Jason Kim (Advisor). Lauren and Allison expressed heartfelt appreciation to the DVSC25 interns for their growing passion and commitment to diabetes research, education, and care. Our interns asked outstanding questions using the Chat box following each session, demonstrating vital traits of an investigator, observation, and inquiry. Their dedication to learning was demonstrated through the pre-session Q&A period and echoed during the open-mic forum. Dr. Kim explained the process of requesting a program certificate for the Diabetes Virtual Summer Camp 2025. The Diabetes Virtual Camp Team then administered electronic polls and surveys to collect important feedback from the interns about the program and explained the final program task to be completed within a week. Our interns didn’t want to leave as we held a final open-mic forum where the interns were unmuted to directly ask questions and provide more feedback on our program. The Closing Sessions ended with the final farewells to everyone as we remain hopeful that our program has met the goals to supporting the next generation of physicians and scientists and one day finding a cure for diabetes. The Diabetes Virtual Camp looks forward to seeing you all in 2026! -
![]()
August 15, 2025 (Session 13 - Part 7)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
After 13 exciting sessions of the Diabetes Virtual Summer Camp 2025, we arrived at the Closing Session with Lauren Kim (Founder, Program Director, and Webmaster), Allison Kim (Associate Director and Director of Outreach), and Dr. Jason Kim (Advisor). Lauren and Allison expressed heartfelt appreciation to the DVSC25 interns for their growing passion and commitment to diabetes research, education, and care. Our interns asked outstanding questions using the Chat box following each session, demonstrating vital traits of an investigator, observation, and inquiry. Their dedication to learning was demonstrated through the pre-session Q&A period and echoed during the open-mic forum. Dr. Kim explained the process of requesting a program certificate for the Diabetes Virtual Summer Camp 2025. The Diabetes Virtual Camp Team then administered electronic polls and surveys to collect important feedback from the interns about the program and explained the final program task to be completed within a week. Our interns didn’t want to leave as we held a final open-mic forum where the interns were unmuted to directly ask questions and provide more feedback on our program. The Closing Sessions ended with the final farewells to everyone as we remain hopeful that our program has met the goals to supporting the next generation of physicians and scientists and one day finding a cure for diabetes. The Diabetes Virtual Camp looks forward to seeing you all in 2026! -
![]()
August 15, 2025 (Session 13 - Part 8)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Lauren Kim (Founder, Program Director, and Webmaster, University of Southern California, B.S. in Human Biology, 24’) -
![]()
August 15, 2025 (Session 13 - Part 9)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Allison Kim (Associate Director and Director of Outreach, Boston University, B.A. in Biology, 23’) -
![]()
August 15, 2025 (Session 13 - Part 10)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Dr. Jason Kim (Professor of Molecular Medicine and Professor of Medicine, Division of Endocrinology, Metabolism, and Diabetes, Director of Metabolic Disease Research Center, MD/PhD Admissions Committee, University of Massachusetts Chan Medical School) -
![]()
August 15, 2025 (Session 13 - Part 11)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
After 13 exciting sessions of the Diabetes Virtual Summer Camp 2025, we arrived at the Closing Session with Lauren Kim (Founder, Program Director, and Webmaster), Allison Kim (Associate Director and Director of Outreach), and Dr. Jason Kim (Advisor). Lauren and Allison expressed heartfelt appreciation to the DVSC25 interns for their growing passion and commitment to diabetes research, education, and care. Our interns asked outstanding questions using the Chat box following each session, demonstrating vital traits of an investigator, observation, and inquiry. Their dedication to learning was demonstrated through the pre-session Q&A period and echoed during the open-mic forum. Dr. Kim explained the process of requesting a program certificate for the Diabetes Virtual Summer Camp 2025. The Diabetes Virtual Camp Team then administered electronic polls and surveys to collect important feedback from the interns about the program and explained the final program task to be completed within a week. Our interns didn’t want to leave as we held a final open-mic forum where the interns were unmuted to directly ask questions and provide more feedback on our program. The Closing Sessions ended with the final farewells to everyone as we remain hopeful that our program has met the goals to supporting the next generation of physicians and scientists and one day finding a cure for diabetes. The Diabetes Virtual Camp looks forward to seeing you all in 2026! -
![]()
August 15, 2025 (Session 13 - Part 12)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
After 13 exciting sessions of the Diabetes Virtual Summer Camp 2025, we arrived at the Closing Session with Lauren Kim (Founder, Program Director, and Webmaster), Allison Kim (Associate Director and Director of Outreach), and Dr. Jason Kim (Advisor). Lauren and Allison expressed heartfelt appreciation to the DVSC25 interns for their growing passion and commitment to diabetes research, education, and care. Our interns asked outstanding questions using the Chat box following each session, demonstrating vital traits of an investigator, observation, and inquiry. Their dedication to learning was demonstrated through the pre-session Q&A period and echoed during the open-mic forum. Dr. Kim explained the process of requesting a program certificate for the Diabetes Virtual Summer Camp 2025. The Diabetes Virtual Camp Team then administered electronic polls and surveys to collect important feedback from the interns about the program and explained the final program task to be completed within a week. Our interns didn’t want to leave as we held a final open-mic forum where the interns were unmuted to directly ask questions and provide more feedback on our program. The Closing Sessions ended with the final farewells to everyone as we remain hopeful that our program has met the goals to supporting the next generation of physicians and scientists and one day finding a cure for diabetes. The Diabetes Virtual Camp looks forward to seeing you all in 2026! -
![]()
August 15, 2025 (Session 13 - Part 13)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Lauren Kim summarizes the program and the process of receiving a program certificate, collects attendee evaluations and polls, and discusses future opportunities. -
![]()
August 15, 2025 (Session 13 - Part 14)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Allison Kim summarizes the program and the process of receiving a program certificate, collects attendee evaluations and polls, and discusses future opportunities. -
![]()
August 15, 2025 (Session 13 - Part 15)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
After 13 exciting sessions of the Diabetes Virtual Summer Camp 2025, we arrived at the Closing Session with Lauren Kim (Founder, Program Director, and Webmaster), Allison Kim (Associate Director and Director of Outreach), and Dr. Jason Kim (Advisor). Lauren and Allison expressed heartfelt appreciation to the DVSC25 interns for their growing passion and commitment to diabetes research, education, and care. Our interns asked outstanding questions using the Chat box following each session, demonstrating vital traits of an investigator, observation, and inquiry. Their dedication to learning was demonstrated through the pre-session Q&A period and echoed during the open-mic forum. Dr. Kim explained the process of requesting a program certificate for the Diabetes Virtual Summer Camp 2025. The Diabetes Virtual Camp Team then administered electronic polls and surveys to collect important feedback from the interns about the program and explained the final program task to be completed within a week. Our interns didn’t want to leave as we held a final open-mic forum where the interns were unmuted to directly ask questions and provide more feedback on our program. The Closing Sessions ended with the final farewells to everyone as we remain hopeful that our program has met the goals to supporting the next generation of physicians and scientists and one day finding a cure for diabetes. The Diabetes Virtual Camp looks forward to seeing you all in 2026! -
![]()
August 15, 2025 (Session 13 - Part 16)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
After 13 exciting sessions of the Diabetes Virtual Summer Camp 2025, we arrived at the Closing Session with Lauren Kim (Founder, Program Director, and Webmaster), Allison Kim (Associate Director and Director of Outreach), and Dr. Jason Kim (Advisor). Lauren and Allison expressed heartfelt appreciation to the DVSC25 interns for their growing passion and commitment to diabetes research, education, and care. Our interns asked outstanding questions using the Chat box following each session, demonstrating vital traits of an investigator, observation, and inquiry. Their dedication to learning was demonstrated through the pre-session Q&A period and echoed during the open-mic forum. Dr. Kim explained the process of requesting a program certificate for the Diabetes Virtual Summer Camp 2025. The Diabetes Virtual Camp Team then administered electronic polls and surveys to collect important feedback from the interns about the program and explained the final program task to be completed within a week. Our interns didn’t want to leave as we held a final open-mic forum where the interns were unmuted to directly ask questions and provide more feedback on our program. The Closing Sessions ended with the final farewells to everyone as we remain hopeful that our program has met the goals to supporting the next generation of physicians and scientists and one day finding a cure for diabetes. The Diabetes Virtual Camp looks forward to seeing you all in 2026! -
![]()
August 15, 2025 (Session 13 - Part 17)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Our interns stay for the final Open Mic Forum as the DVC team invites our interns to ask questions and engage in a productive discussion about the program. -
![]()
August 15, 2025 (Session 13 - Part 18)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Our interns stay for the final Open Mic Forum as the DVC team invites our interns to ask questions and engage in a productive discussion about the program. -
![]()
August 15, 2025 (Session 13 - Part 19)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Our interns stay for the final Open Mic Forum as the DVC team invites our interns to ask questions and engage in a productive discussion about the program. -
![]()
August 15, 2025 (Session 13 - Part 20)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Our interns stay for the final Open Mic Forum as the DVC team invites our interns to ask questions and engage in a productive discussion about the program. -
![]()
August 15, 2025 (Session 13 - Part 21)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Our interns stay for the final Open Mic Forum as the DVC team invites our interns to ask questions and engage in a productive discussion about the program. -
![]()
August 15, 2025 (Session 13 - Part 22)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
Our interns stay for the final Open Mic Forum as the DVC team invites our interns to ask questions and engage in a productive discussion about the program. -
![]()
August 15, 2025 (Session 13 - Part 23)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
The Diabetes Virtual Camp Team says “goodbyes” to our DVSC25 interns as we look forward to seeing you all in 2026! -
![]()
August 15, 2025 (Session 13 - Part 24)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
The Diabetes Virtual Camp Team says “goodbyes” to our DVSC25 interns as we look forward to seeing you all in 2026! -
![]()
August 15, 2025 (Session 13 - Part 25)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
The Diabetes Virtual Camp Team says “goodbyes” to our DVSC25 interns as we look forward to seeing you all in 2026! -
![]()
August 15, 2025 (Session 13 - Part 26)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
The Diabetes Virtual Camp Team says “goodbyes” to our DVSC25 interns as we look forward to seeing you all in 2026! -
![]()
August 15, 2025 (Session 13 - Part 27)
Session 13. Closing Session by the DVC Team (Lauren Kim, Allison Kim, and Dr. Jason Kim)
The Diabetes Virtual Camp is supported in part by the American Diabetes Association and The Leona M. and Harry B. Helmsley Charitable Trust. We are grateful for their support of our important mission, inspiring our next generation of physicians and scientists.